William A. Shear1, Tappey H. Jones2, Thomas Wesener3
Introduction
Nearly simultaneously in 1966, Schildknecht et al. (1966)and Meinwald et al. (1966) announced the discovery of two quinazolinone alkaloids in the defensive secretion of the European millipede Glomeris marginata (Villers, 1789). Schildknecht et al. (1966, 1967) named one of these compounds glomerin; later Schildknecht and Wenneis (1966)
were able to associate the name glomerin with a specific structure: 1,
2-dimethyl-4(3H)-quinazolinone, a result confirmed independently by Meinwald et al. (1966). The second compound, homoglomerin, is 1-ethyl-2-methyl-4(3H)-quinazolinone (Schildknecht and Wenneis 1967a). Up to the time of this study, Glomeris marginata was the only known animal source of any quinazolinone alkaloid (Eisner et al. 2005). Schildknecht and Wenneis (1967b) established by radioactive tracer studies that Glomeris marginata synthesizes quinazolinones from anthranilic acid and does not sequester them from plant sources.
Both compounds were found to be potent repellents of frogs, birds and mice (Schildknecht and Wenneis 1967a) and to act as antifeedants and sedatives to a lycosid spider (Carrell and Eisner 1984).
Carrell (1984)
studied the production and storage of the secretion, which, in
addition to its content of glomerin and homoglomerin, contains
protienaceous components that make it very sticky, thus adding a
physical dimension to its protection for the millipede. Following this
1984 publication, no further research on the occurrence of glomerin and
homoglomerin was published. Summaries appear in Eisner et al. (1978, 2005).
Glomerid millipedes are capable of complete enrollment.
The posteriormost body segment, the anal shield, is enlarged and
hoodlike, and in the enrolled condition completely covers the head and
much-reduced collum (first postcephalic segment), locking under the
anterior margin of the second segment, while the semispherical,
overlapping terga enclose the sides (Hopkin and Read 1992,
and pers. obs. by WAS). Thus a potential predator such as a spider or
ant is presented with a hard, glossily smooth, and nearly perfect
sphere. However, large wolf spiders are capable of spanning small
enrolled glomerids with their chelicerae, and biting them (Carrell and Eisner 1984),
and one presumes that frogs, birds or mice could swallow such morsels
whole. Despite the protection against some enemies provided by the hard
exoskeleton and enrolling habit, Glomerida are the only order of the
millipede subclass Pentazonia which additionally possess chemical
defenses (Sierwald and Bond 2007). Beginning on the third segment and continuing posterior for eight segments (to the antepenultimate), Glomeris
species bear dorsomedian pores (the so-called ozopores) from which
empty the products of paired, or two-armed repugnatorial glands; these
glands produce the sticky secretion that also contains glomerin and
homoglomerin. The secretion is expelled by muscles wound around the
glands, through a muscular valve (Eisner et al. 1978). In keeping with this potent chemical weapon, many species of Glomeris are aposematically colored, with transverse bands or spots of bright yellow, orange or red (Blower 1958, Demange 1981, pers. obs. by WAS).
While a prominent feature of the soil fauna of much of
Europe, only two of the 34 described glomerid genera are found in
America, where they appear at present to be limited to the southern
Appalachian Mountains (Onomeris) and to the San Francisco Bay area of California, southern México, and Guatemala (Glomeroides) (Shear 1986, Hoffman 1999). Onomeris sinuata was originally described by Loomis (1943) from Monte Sano State Park, Alabama, as Trichomeris sinuata; Trichomeris was synonymized with Onomeris by Wesener (2010). Unlike European Glomeris species, species of Onomeris and Glomeroides
are small (4–10 mm long), inconspicuous, dun-colored inhabitants of
soil and deciduous leaf litter, lacking any aposematic coloration. A
number of Glomeroides species have so far been recorded only from Central American and Méxican caves (Shear 1986). Neither chemical defense nor ozopores have been previously demonstrated in any North American species.
Methods
Specimens of Onomeris sinuata
were collected by TW at the type locality, Monte Sano State Park,
Alabama, under a permit issued by the state of Alabama. Specimens were
placed in containers with damp leaf litter from the collection site,
transported to the Field Museum of Natural History in Chicago and
subsequently to the laboratory of WAS in Hampden-Sydney, Virginia.
There, the millipedes were subjected to various physical stresses
designed to elicit secretion and finally were live-extracted in small
quantities of methanol in glass vials with Teflon-lined caps. The vials
were sent to the laboratory of THJ at the Virginia Military Institute,
Lexington, Virginia, for analysis.
Gas Chromatograph/Mass Spectroscopy analysis of the
methanol extract was carried out using a Shimadzu model 2010 GC/MS
equipped with an RTX-5, 30 m 3 0.25-mm i.d. The specimens are now
preserved in 70% ethanol and eventually will be deposited as vouchers in
the collection of the Field Museum.
Additional specimens (FMNH-INS 56316) preserved in 95%
ethanol were carried through a dehydrating series of alcohols,
air-dried overnight and sputtered for 240 seconds with gold with a
Denton Vacuum Desk IV. Examinations were done on a Zeiss (Leo) EVO
scanning electron microscope based at the Field Museum of Natural
History, Chicago.
Results
Live specimens of Onomeris sinuata
were subjected to several kinds of physical stress twice daily over a
four-day period. Animals were removed from the leaf litter in which they
were kept and placed in a small, rectangular plastic arena floored
with damp paper toweling. Transfer was accomplished using a plastic
spoon to avoid disturbing the millipedes. After two minutes of recovery
time, the animals began freely exploring the arena. They were tapped
lightly with the head of an insect pin, which induced them to roll up
as described above. The enrolled animals were then lightly squeezed with
padded forceps. It was possible to quickly grasp walking millipedes by
one or more legs, which also caused them to enroll, often around the
tips of the forceps. Individuals were dropped into small vials and
shaken. None of these disturbances caused any visible secretion that
could be observed at 20X magnification under a dissecting microscope. In
contrast, the secretion of Glomeris marginata is easily elicited and clearly visible (Carrell and Eisner 1984, Eisenbeis and Wichard 1987 [their fig. 88b], pers. obs. by TW).
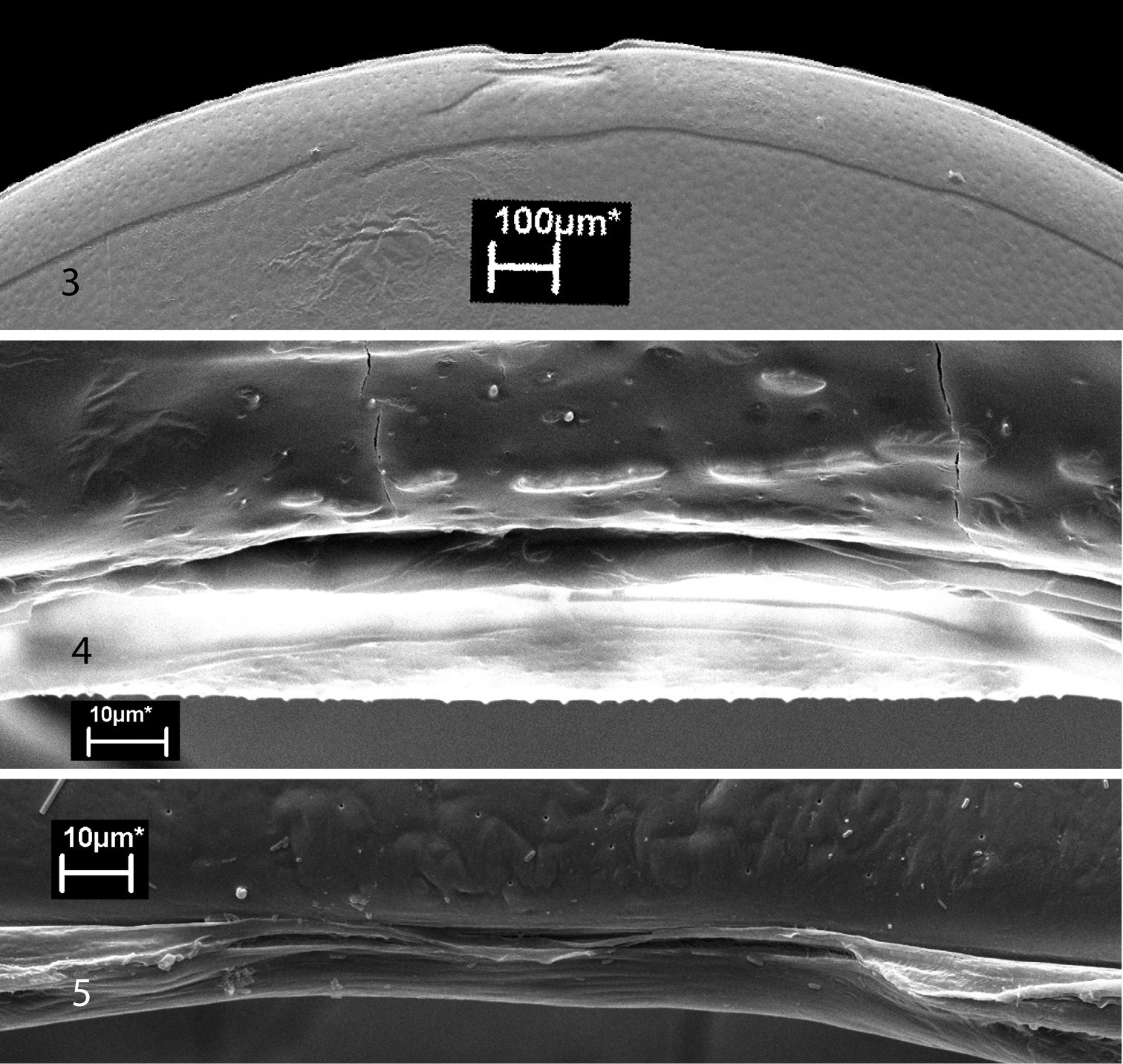
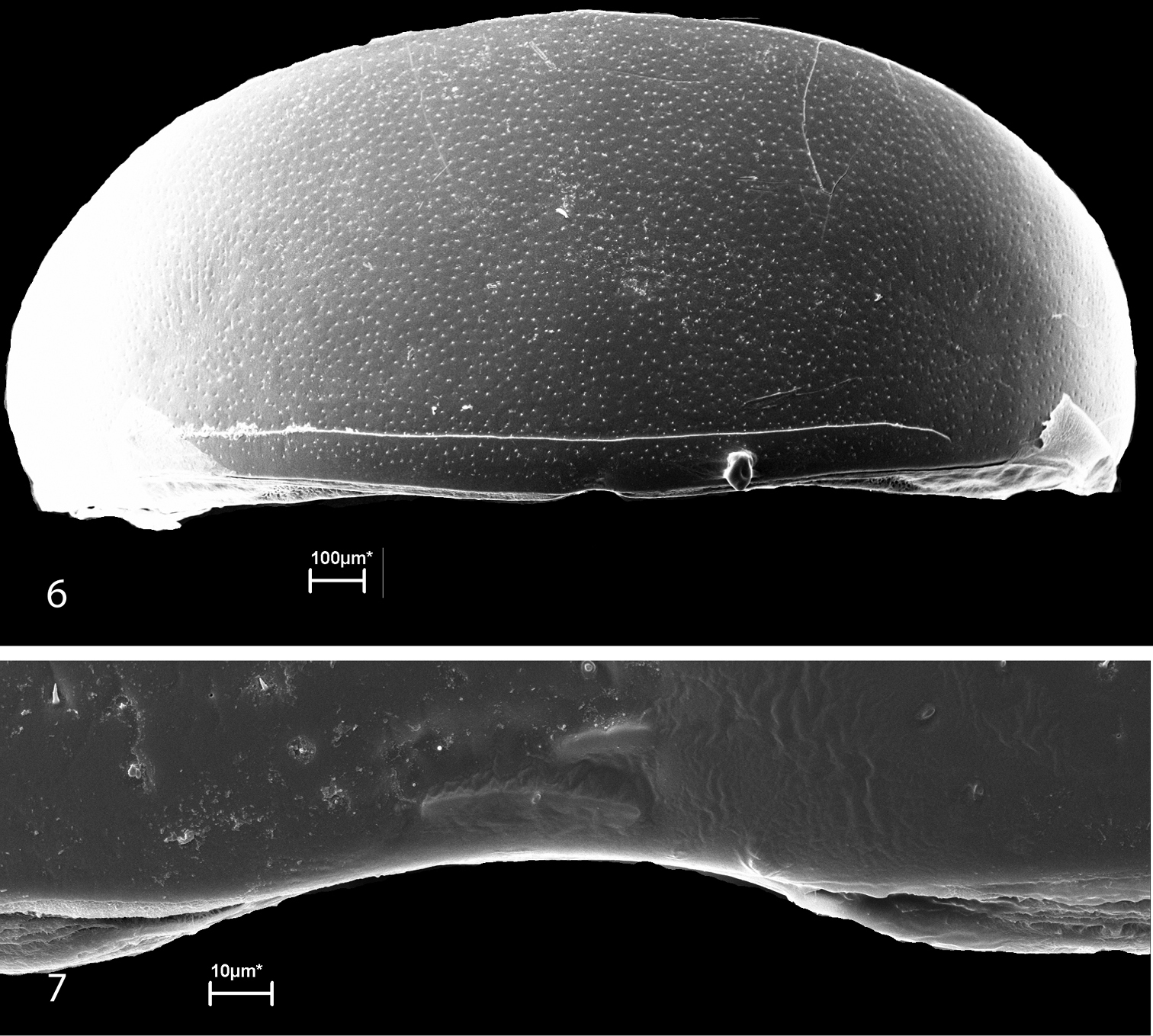
Scanning electron micrographs of the ozopores of glomerids have never been published. As depicted by Verhoeff (1926),
the inconspicuous pores are actually located in the intersegmental
membranes immediately behind the posterior margins of the diplotergites.
There is a slight modification on the anterior margin of the adjacent
diplotergite margin near the opening of the gland. These modifications (Figs 3–7), which are more pronounced in Glomeris marginata (Fig. 3), were interpreted as helping in the spread of the secretion along the diplotergite (Verhoeff 1926). In our illustrations, the pore itself appears to be double, or at least somewhat constricted in the midline.
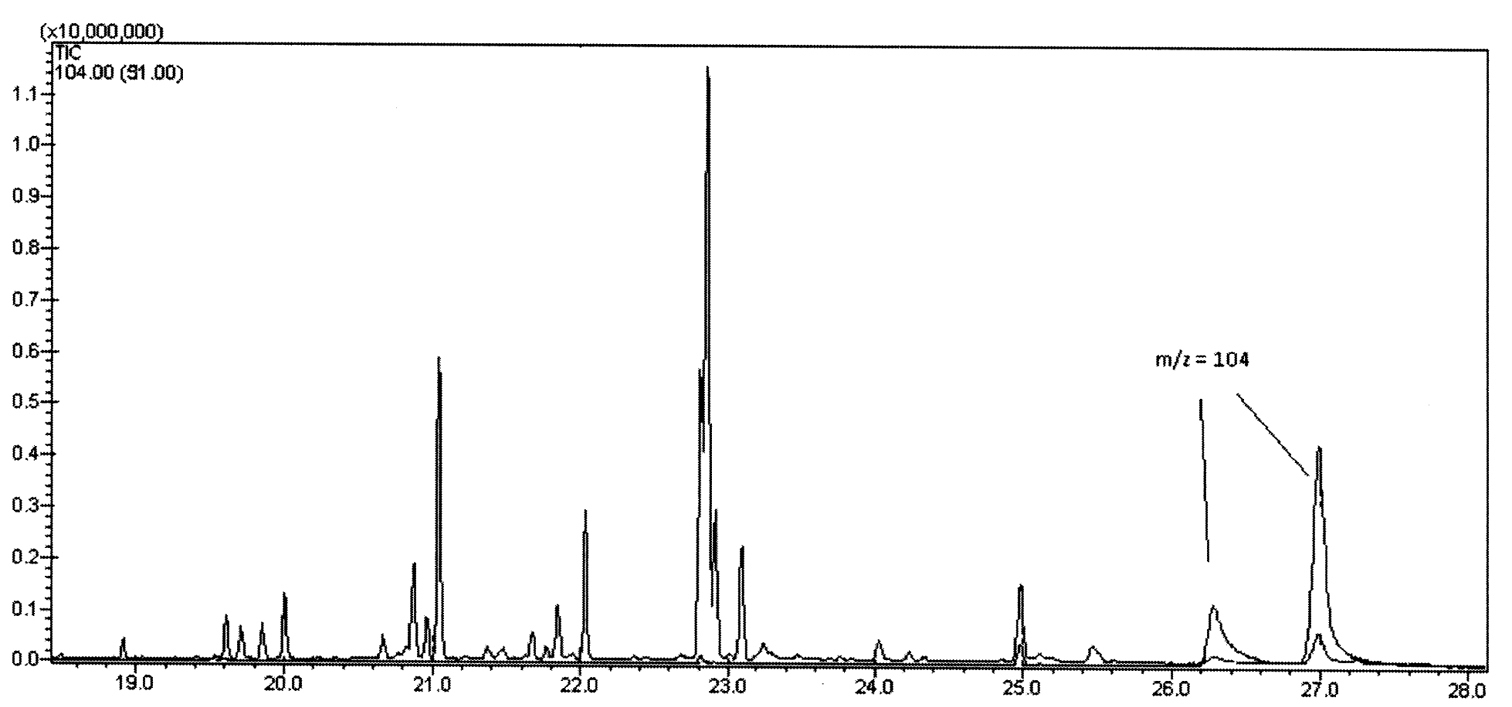
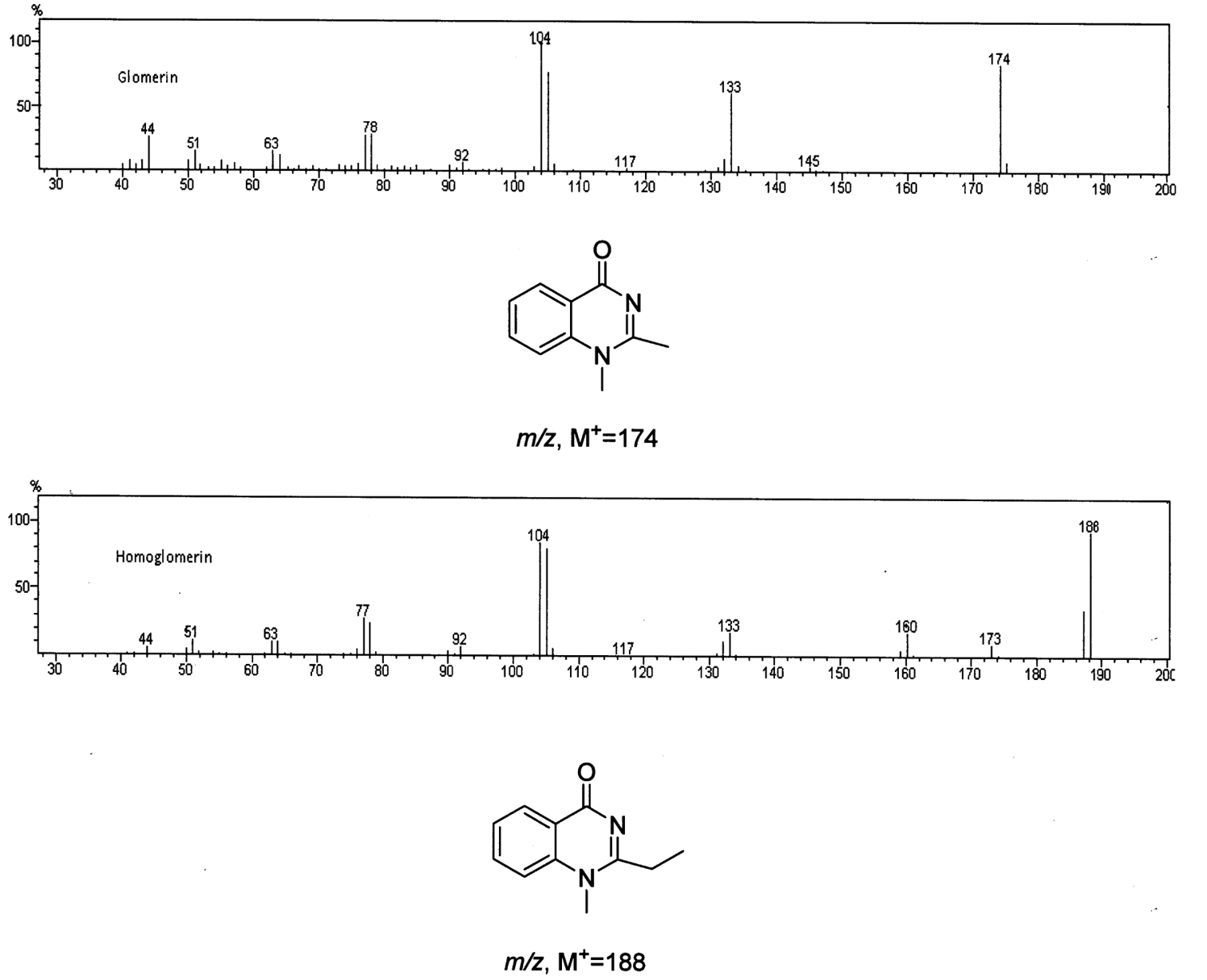
GC/MS analysis of the methanol extracts, scanning for a common fragment at m/z = 104, revealed the presence of twoquinazolinones in a 1:4 ratio (Fig. 1). Their mass spectra (Fig. 2) matched those reported for glomerin and homoglomerin (Meinwald 1966, Schildknecht and Wenneis 1967a).
Additionally, a number of fatty acids were detected as their methyl
esters (FAMES). Along with the usual C-16 and C-18 FAMES at 20.6 to 21.5
min and 22.7–23.1 min respectively, several C-15 and C-17 FAMES were
detected from 19.6 to 20.0 min and 21.6 to 22.1 min respectively (Fig. 1).
Based on previous knowledge we assume the quinazolinone alkaloids came
from the repugnatorial glands, and the fatty acids likely from fat
tissues in the millipedes’ bodies.
Figure 1.
Gas Chromatogram of the extract of Trichomeris sinuata showing the m/z =104 characteristic of Glomerin and Homoglomerin.
Figure 2.
Mass spectra and structures of Glomerin and Homoglomerin respectively.
Discussion
The illustrations in Eisner et al.(1978) are erroneous in
showing the pores opening from within the diplotergite. While in their
text, Eisner et al. (1978)
state that the glands open on segments 4–11, their drawings and
photographs clearly show that the glands open just behind segments 3–10.
Verhoeff (1928, p. 1030) states, quoting Latzel (1884):
“Das erste Saftloch liegt an der Basis des 4. Rückenschildes.” If we
take one meaning of “Basis” it is suggested that the pores open anterior
to the fourth diplotergite, and it is clear from the position of the
glands and the modification of the anterior margin of the fourth
diplotergite that the glands and the pore actually belong to the third
and hence should be considered as opening on segments 3–10, not 4–11.
While the illustrated pore of Glomeris marginata appears single, the pore of Onomeris sinuata seems at least to be constricted in the middle, and that of Glomeroides primus
(Silvestri, 1929) appears to be bilaterally double, perhaps
reflecting the dual nature of the glands. It may be that in the
ancestors of the glomerids there were two pores, more lateral in
position, serving two separate glands. As the ability to enroll
developed the pores and glands may have moved dorsally and partially or
entirely fused. Eisenbeis and Wichard (1987)
suggest that the middorsal position of the glands is optimal in
providing coverage for the enrolled animal. On the other hand, the
unique position and structure of the glands, the unusual chemistry of
the secretion and its sticky nature, not seen in any helminthomorph
millipedes, could be interpreted as supporting a separate evolutionary
origin for chemical defense in glomerids. The other two orders of
Pentazonia (Glomeridesmida, Sphaeriotheriidae) lack repugnatorial
glands and pores.
Given that no secretion could be elicited in the
laboratory, we were surprised at the results of the analysis of the
extract. Our sample size in the “stress test” is small (4 individuals),
but we speculate that some stimulus other than physical disturbance may
be needed to trigger the production of secretion. It is likely that
ants are major predators of these small, soil and litter-dwelling
glomerids, and it may be that some chemical clue associated with ant
predation may elicit secretion.
The effectiveness of the quinazolinone alkaloids against ants has never been tested, but the stickiness of the Glomeris marginata secretion has been observed to “quickly immobilize” ants (Eisner et al. 2005). However, quinazolinones cause vomiting in toads, partial hypnosis in birds, and partial paralysis in mice (Schildknecht and Wenneis 1967a). Carrell and Eisner (1984)
found that glomerin, and especially homoglomerin, were active as
antifeedants against wolf spiders, but at a much lower dosage than was
needed to completely sedate the spiders of the same size; in fact,
spiders which rejected Glomeris marginata
without killing or feeding on the millipedes often became sedated. That
spiders (and perhaps other arthropods) might be the targets of
glomerins is suggested by the fact that closely related compounds such
as arborine, a quinazolinone from a plant, and methaqualone, a
synthetic sedative, do not sedate spiders (Carrell et al. 1984). Both are sedative to vertebrates (Dey and Chatterjee 1967, Inaba et al. 1973).
However, it is worth noting that Glomeris marginata is native to Europe (see Heath et al. 1974 for data on life history), while the spider used in predation tests by Carrell and Eisner (1984) and in sedation studies by Carrell et al. (1984), Hogna ceratiola
(Gertsch & Wallace, 1935), is endemic to scrub habitats in
central Florida, USA. The effects of glomerins have not been tested
against potential invertebrate predators of Glomeris marginata in its native habitat, though lycosid spiders large enough to attack Glomeris marginata occur there.
In keeping with its dark, obscure habitat, Onomeris sinuata
shows no signs of aposematic coloration and probably is not a target of
visually hunting predators, as it rarely appears above the surface of
the leaf litter. Our live specimens consistently fled light and
concealed themselves in available litter. Aposematism is indicated for
many Glomeris species, with classic coloration in shiny black offsetting bright yellow, orange or red spots and bands, though Glomeris marginata is not obviously aposematic compared to congeners such as Glomeris humbertiana Saussure, 1893 or Glomeris connexa C.L. Koch, 1847 (Demange 1981, see color plate III). Carrel and Eisner (1984) and Carrellet al. (1984) speculated on the significance of supposed aposematism in Glomeris marginata.
Typically, aposematic prey survives a predatory attack and the
predator becomes “educated” in the avoidance of similar prey. However, Glomeris marginata individuals were often killed by Hogna ceratiola
spiders before the spider dropped them or became sedated. Further, the
sedation would almost always be fatal to the spiders in nature because
they would become vulnerable to their own predators, especially to the
ever-present ants (Eisner et al. 2005
picture such a spider under attack by ants). Thus Carrell and Eisner
questioned how aposematism would work in this system, eventually
suggesting that since millipedes have limited mobility, members of the
same species in the same area may be related, and therefore the
“sacrifice” of some individuals in the population could help relatives
that carry some of the same genes. There are two flaws in this scenario.
One is that there has been no demonstration that even spiders that
survive an encounter with Glomeris marginata
have been conditioned at all; in fact learning by spiders is very
limited. We think it more likely that the rather poorly marked
aposematism of Glomeris marginata is directed to visually hunting vertebrate predators in its native habitat. The second flaw, of course, is that Hogna ceratiola could not be a natural predator of Glomeris marginata
because the two species occur an ocean away from each other. Thus tests
involving this spider have only a suggestive significance.
More work is required to understand the significance of the presence of glomerin and homoglomerin in Onomeris sinuata
secretions, especially to determine likely predators of the millipede
and the effects, if any, of the secretion against them. We also need
to understand why the millipedes seem so reluctant to produce their
chemical defense when treated roughly in a way that immediately would
cause other species to do so.
Figures 3–5.
Ozopores of glomerids. 3 Tergite 4 of Glomeris marginata, anterior above; note modified tergal margin above ozopore 4 Ozopore of Glomeris marginata 5 Ozopore of Onomeris sinuata; tergal margin is unmodified.
Figures 6–7.
Ozopores of Glomeroides primus. 6 Tergite 4, anterior below; note modified tergal margin above ozopore 7 Ozopore.