(C) 2011 Amazonas Chagas-Júnior. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The neotropical genus Tidops Chamberlin, 1915 is reviewed and its geographical distribution is extended. The genus is now composed of four species – Tidops simus Chamberlin, 1915, Tidops collaris (Kraepelin, 1915), Tidops balzanii (Silvestri, 1895), and a new species described from the state of Bahia, in Brazil, named as Tidops nisargani. An amended diagnosis for each species is given. The monotypic genus Kartops Archey, 1923 was examined for the first time and the species Kartops guianae Archey, 1923 shown to be conspecific with Tidops collaris, and is therefore a junior synonym of Tidops collaris. The type species, Tidops simus, which was only known from Grenada, Lesser Antilles, is recorded for the first time from Brazil, Tidops balzanii from Bolivia and Tidops collaris from Venezuela, respectively.
Chilopoda, Newportia, Kartops, Taxonomy, Neotropical, Brazil
Tidops is a Neotropical genus of scolopendromorph centipedes described by
Repository acronyms are as follows: AMNH—American Museum of Natural History, New York, New York, USA; BMNH—Natural History Museum, London, England; IBSP—Instituto Butantan, São Paulo, São Paulo, Brazil, MCZ—Museum of Comparative Zoology, Harvard University, Cambridge, Massachusetts, USA; MNRJ—Museu Nacional, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil; MNHN—Muséum National d´Histoire Naturelle, Paris, France; MPEG—Museu Paraense Emilio Goeldi, Belém, Pará, Brazil; NMNH—National Museum of Natural History, Smithsonian Institution, Washington, D. C., USA; ZMH—Zoologisches Museum Hamburg, Hamburg, Germany; ZMUC—Natural History Museum of Denmark (Zoologisk Museum), Copenhagen, Denmark and CMNZ—Canterbury Museum, Christchurch, New Zealand.
Descriptive terminology follows
Family Scolopocryptopidae Pocock, 1896
Median-sized to small scolopocryptopids, general body color brown, dark or pale yellow, cephalic plate reddish brown and legs yellow. Cephalic plate, tergites and sternites normally smooth, with or without short incomplete sutures starting on the posterior border of the cephalic plate; with or without a pair of spiracles on the seventh pedal segment, last tergite always margined, sternites usually longer than wide, with coxopleural processes, ultimate legs elongated and relatively slender, prefemora of the ultimate legs with three or more and femur and tibia with or without spinous processes. Second tarsus divided in “pseudoarticles”.
Newportia and Tidops.
Neotropical.
http://species-id.net/wiki/Tidops
Tidops simus Chamberlin, 1915 (by monotypy).
Small Newportiinae, cephalic plate with two short incomplete sutures at the posterior border; forcipular coxosternum with short tarsungula, only the apices overlap in closed position; coxosternal tooth plates dentiform; 7th pedal segments without spiracles; first tergite with anterior transverse sulcus giving rise to paired furcate sutures extending posteriorly in a “W-shaped” configuration, with two paired sutures arising from base of former, angling slightly laterally to posterior margin; tibia of the ultimate legs without spines or spinous process, very much thicker than the tarsus 1, somewhat clavately widening distad and at ventral corner of distal end bearing a cylindric process; tarsus 1 with or without a distal ventral expansion.
Tidops simus, Tidops collaris, Tidops balzanii and Tidops nisargani sp. n.
Grenada, Guyana, Venezuela (new record), Brazil, Bolivia (new record) and Paraguay.
The presence or absence of a pair of spiracles on the seventh pedal segment is traditionally used in the diagnosis of some genera of Scolopendromorpha.
http://species-id.net/wiki/Tidops_simus
Holotype (MCZ 14550, examined) GRENADA: Richmond Hill, 1910, coll. G. M. Allen and C. T. Brues.
(ZMUC) 1 specimen, BRAZIL: “Brasilien”, Pará, Santarém, Taperinha, 9-XI-1970, Ove Jensen.
Prefemur of the ultimate legs with three ventral spinal processes, and femur with two. With a cylindric process at ventral corner of distal end of the tibia of the ultimate legs. Tarsus 1 of the ultimate legs clavate. (cf.
The holotype of Tidops simus is mounted on a slide and almost all pieces are broken. The specimen has been mounted ventrally and it is not possible to examine characters of the dorsal part and the legs, which are missing. The only relevant character which could be observed was the forcipular coxosternum. I have found a specimen of Tidops from Brazil that was assigned as Tidops simus mainly on the characters of the ultimate legs and the tooth plates (i.e., their diagnostic length/width ratio). The prefemur of the ultimate legs has a series of three ventral spinous processes and the femur one or two. The tibia has a cylindric process at ventral corner of distal end and tarsus 1 clavate as described to the holotype of Tidops simus by
Richmond Hill, Grenada.
Grenada and Brazil (new record).
http://species-id.net/wiki/Tidops_balzanii
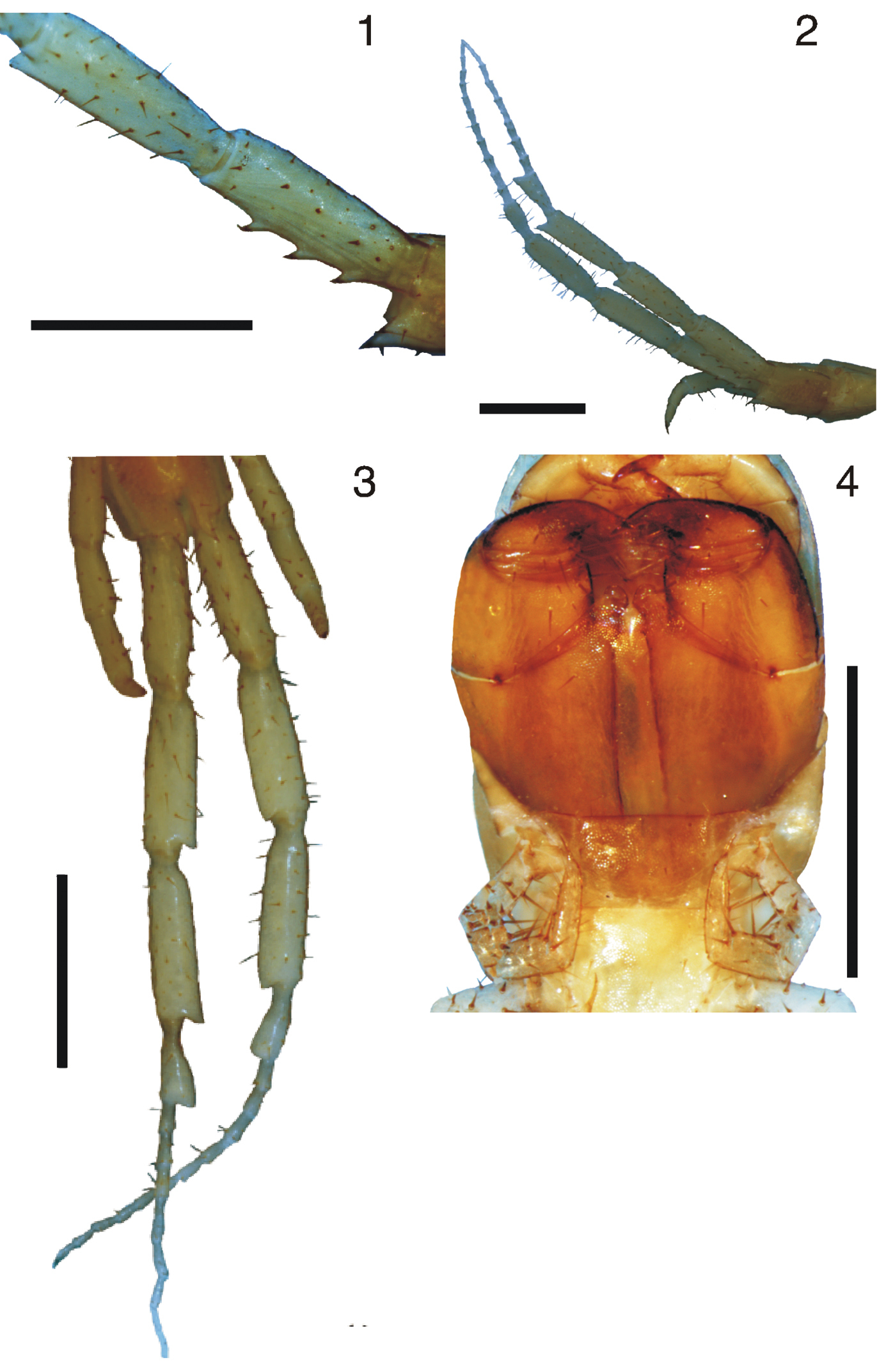
Figures 1–4(BMNH) 1 specimen, Guyana-Brazil: 19-VII-1937, H. E. Hinton; (IBSP 1546) 1 specimen, BRAZIL: Tocantins, Palmas, UHE. Luis Eduardo Magalhães, Margem direita TO-10, Km1.5, 03-IX-2001, I. Knysak, R. Martins & G. Puorto; (IBSP 1559) 1 specimen, Tocantins, Palmas, UHE. Luis Eduardo Magalhães, 30-XI-2000, I. Knysak, R. Martins & G. Puorto; (IBSP 2783) 2 specimens, Tocantins, Porto Nacional, I. Knysak, R. Martins & G. Puorto; (IBSP 751) 1 specimen, Goiás, Aragarças, 18-III-1953, H. Sick; (MNRJ 15303) 10 specimens, Goiás, Jandaia, Fazenda Sr. João Batista Ferreira, 04-VI-2007, A. Chagas, A. Giupponi, A. Kury & A. Pérez; (MNRJ 15356) 3 specimens, Goiás, Paraúna, 30-XII-2006, Chagas-Jr, A., Segal, B. & Chagas, C. S.; (IBSP 710) 1 specimen, São Paulo, Pirassununga, 10-VI-1952, O. Schubart; (IBSP 704) 1 specimen, São Paulo, Pirassununga (Baguassu), 10-VI-1952, O. Schubart; (IBSP 93) 1 specimen, São Paulo, Corumbataí, 02-X-1933, S. Burian; (IBSP 180) 1 specimen, São Paulo, Amparo, 10-VI-1944, A. das Neves; (NMNH) 2 specimens, Rio Grande do Sul, Jaguarão, 28-I-1963, C. S. Carbonell leg.; (NMNH) 3 specimens, BOLIVIA: Huachi, W. M. Mann; (ZMH) 1 specimen, PARAGUAY, Puerto Max, Paraguay river, 9-III-1905.
Prefemur of the ultimate pair of legs with three ventral spinous processes (Figure 1); femur and tibia each without ventral spinous process (Figure 2); femur and tibia each with a small cylindric process at ventral corner of distal end and tarsus 1 clavate (Figure 3). Tarsus 2 with 11 or 12 “pseudoarticles”. Tibia longer than or equal to femur. Tooth plates short, dentiform wider than long (Figure 4). Coxopleural process medium to short.
1 Tidops balzanii (IBSP 1559). Prefemur and femur of the ultimate leg showing ventral spinous processes of the prefemur. Lateral view 2 Tidops balzanii (IBSP 1559). Ultimate legs. Lateral view 3 Tidops balzanii (IBSP 1559). Ultimate legs. Ventral view 4 Tidops balzanii (IBSP 1559). Forcipules. Ventral view. Scale bar 1 mm.
The legs of Tidops balzanii are hirsute; articles of the ultimate legs are usually shorter than in the other three species of the genus.
A specimen at the Zoologisches Museum Hamburg, Hamburg, Germany is labeled as “Type Mus. Turin, Argentina: Rio Apa, (C-VI-1903)” and was identified as Newportia balzanii. I am not sure if this specimen corresponds to the holotype of Newportia balzanii.
Type locality. Rio Apa, Paraguay.
Brazil (Tocantins, Goiás, São Paulo and Rio Grande do Sul), Bolivia (new record) and Paraguay.
http://species-id.net/wiki/Tidops_collaris
Figures 5–7Holotype (ZMH, examined) 1 specimen, Bas Carsevenne, (Mus. Paris, C 26-VII-03); paratypes (MNHN, examined) 4 specimens, Bas Carsevenne, 1889, F. Geay.
Types of junior subjective synonyms: Tidops echinopus, holotype (MCZ 14320, examined), GUYANA: Sand Hill Forest, 2nd Mourie, 19.VIII, 1914, coll. F. M. Gaige; paratypes (MCZ, examined) 3 vials; Newportia bicegoi, types (MNHN DCCCIXXI, DCCCLXXI, examined) 3 specimens, BRAZIL: Amazonas, "Manaos", Bicego leg.; Kartops guyanae, holotype (CMNZ, examined), GUYANA, Kartabo, Bartica District, 1920.
Additional material: (NMNH 31269) 4 specimens, GUYANA: Kartabo, Bartica District, 1922 and 17-IV-1924; (NMNH 31975) 6 specimens, Kartabo, Bartica District, 1922 and 8-V-1924; (MNRJ 15355) 2 specimens, VENEZUELA: Edo. Amazonas, Tobogan de La Selva, XII-2002, Pérez-González, A., Giupponi, APL. & Manzanilla, O.; (AMNH) 1 specimen, BRAZIL: Amazonas, Manaus, T. Gilliard; (ZMUC) 3 specimens, Pará, Santarém, Taperinha, 9-11-1970, Ove Jensen; (MCZ 31123) 3 specimens, Pará.
Prefemur of the ultimate legs with four larger ventral spinous processes (Figure 5). Femur with two ventral and one medial spinous process, and tibia without (Figure 6). Femur and tibia without cylindric process at ventral corner of distal end; Femur equal to or a little longer than the tibia. Tarsus 1 not clavate. Tarsus 2 with 21 “pseudoarticles”. The tooth plates short (Figure 7), with the lateral side higher than the medial side, but differing from Tidops simus in being wider than long. Coxopleural process median to long.
5 Tidops collaris (MCZ 31123). Ultimate legs showing the ventral spinous processes of the prefemur and the 2 ventral and 1 median spinous processes of the femur. Ventral view 6 Tidops collaris (MCZ 31123). Ultimate legs. Ventral view 7 Tidops collaris (MCZ 31123). Forcipules Ventral view. Scale bar 1 mm.
The obscure and monotypic genus Kartops, that appears in a clade with Newportia and Tidops in Schileyko and Pavlinov (1997), was here examined for the first time since its description. Kartops was proposed by
Bas Carsevenne, French Guyana (this locality now belongs to Amapá State, in Brazil).
French Guyana (
urn:lsid:zoobank.org:act:
http://species-id.net/wiki/Tidops_nisargani
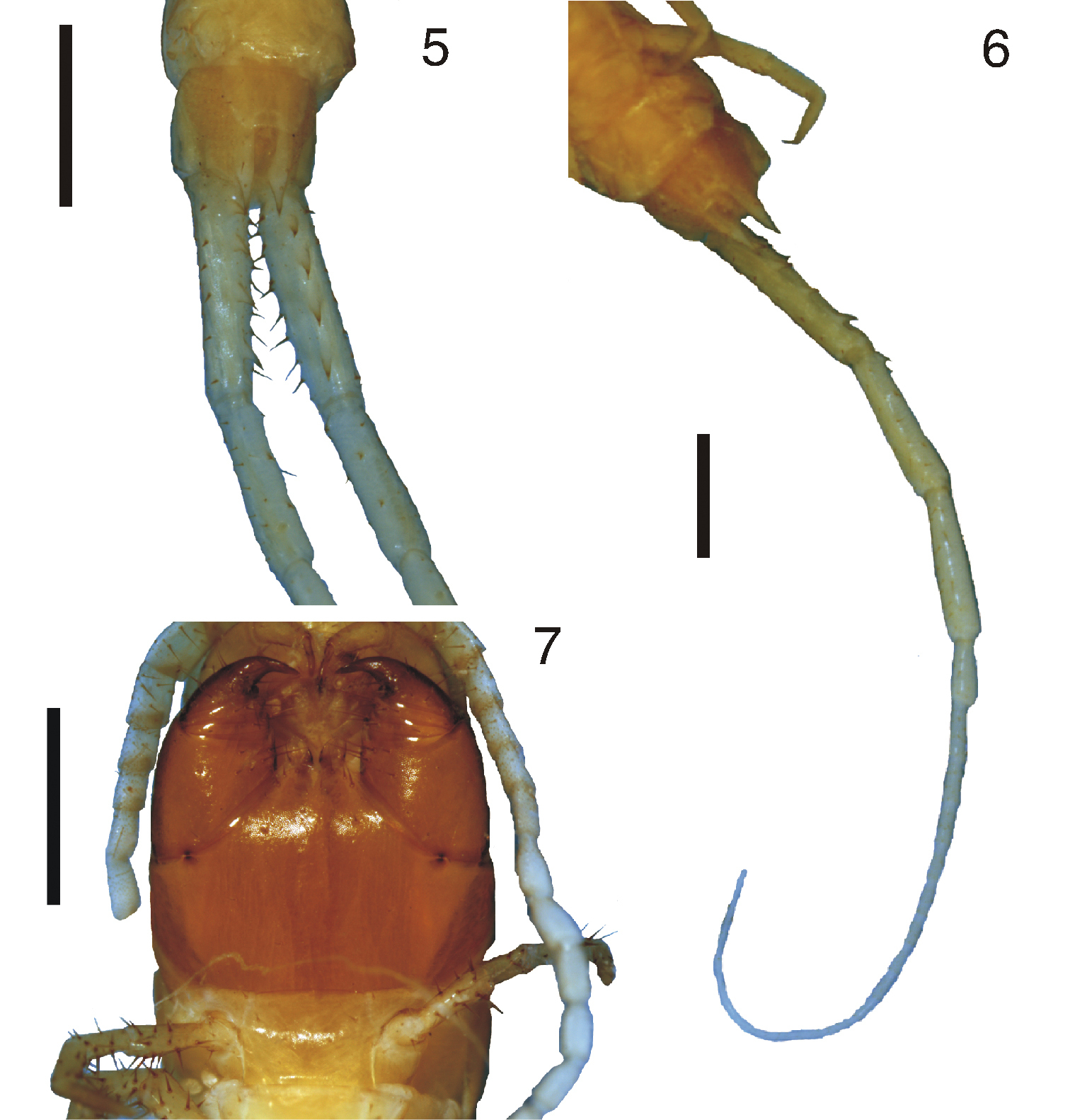
Figures 8–23Holotype (MNRJ 15350), BRAZIL, Bahia, Porto Seguro, Arraial d’Ajuda, 2006 (without specific day and month of collect), Chagas-Jr, A.; Paratypes (MNRJ 15351) 4 specimens, Bahia, Porto Seguro, Arraial d’Ajuda, 2005, Chagas-Jr, A. & Segal, B.; (MNRJ 15499) 7 specimens, Bahia, Porto Seguro, Arraial d’Ajuda, 24-27-ii-2005, Expedição Arachné; (MNRJ 15349) 6 specimens, Bahia, Porto Seguro, Trancoso, 19-vi-2005, Chagas-Jr, A., Segal, B. & Vasconcelos, E.
Additional material. BRAZIL: (MNRJ 15353) 1 specimen, Amazonas, Manaus, i-2001, Pedroso, D.; (MPEG) 6 specimens, Pará, Marabá, Benfica, 15-iii-2003/25-iv-2003, J. Mathieuz & M. Martins; (MNRJ 15352) 11 specimens, Bahia, Alcobaça, Fazenda Santa Bahia, 27-28-II-2005, Expedição Arachné; (MNRJ 15354) 9 specimens, Bahia, Porto Seguro, Arraial D’Ajuda, 24-27-II-2005, Expedição Arachné; (MNRJ 15509) 3 specimens, Bahia, Una, REBIO de Una, 08-10-vi-2009, Chagas-Jr, A., Kury, A., Pedroso, D., Giupponi, A. & Dill, V.; (MNRJ 15510) 1specimen, Bahia, Jequié, Brejo Novo, 06-07-vi-2009, Chagas-Jr, A., Kury, A., Pedroso, D., Giupponi, A. & Dill, V.; (MNRJ 15511) 3 specimens, Bahia, Camacan, RPPN Serra Bonita, 11-13-vi-2009, Chagas-Jr, A., Kury, A., Pedroso, D., Giupponi, A. & Dill, V.; (MNRJ 15512) 3 specimens, Bahia, Una, REBIO de Una, 08-10.vi.2009, Chagas-Jr, A., Kury, A., Pedroso, D., Giupponi, A. & Dill, V.; (MNRJ 15513) 3 specimens, Bahia, Porto Seguro, Parque Nacional do Pau Brasil, 14-17-vi-2009, Chagas-Jr, A., Kury, A., Pedroso, D., Giupponi, A. & Dill, V.; (MNRJ 15514) 3 specimens, Bahia, Porto Seguro, Parque Nacional do Pau Brasil, 14-17-vi-2009, Chagas-Jr, A., Kury, A., Pedroso, D., Giupponi, A. & Dill, V.; (MNRJ 15515) 8 specimens, Bahia, Camacan, RPPN Serra Bonita, 11-13-vi-2009, Chagas-Jr, A., Kury, A., Pedroso, D., Giupponi, A. & Dill, V.; (MNRJ 15516) 1 specimen, Bahia, Camacan, RPPN Serra Bonita, 11-13-vi-2009, Chagas-Jr, A., Kury, A., Pedroso, D., Giupponi, A. & Dill, V.
The specific epithet refers to the “Sítio Nisargan” in Arraial d’Ajuda, Bahia, where the new species was collected and where I lived from 2004 to 2006.
Prefemur of the ultimate legs with four larger spinous processes, femur and tibia without ventral and medial spinous processes; femur and tibia without cylindric process at ventral corner of distal end, but tarsus 1 with a small expansion at ventral corner of distal end; tibia shorter than femur; tarsus 2 with 9 to 15 “pseudoarticles”. The tooth plates longer than wide. The anterior margin of the tooth plates is straight with the lateral sides longer than the medial sides; the inner face of each dentiform tooth plate is concave like a longitudinal section of a cylinder. Coxopleural process moderately long
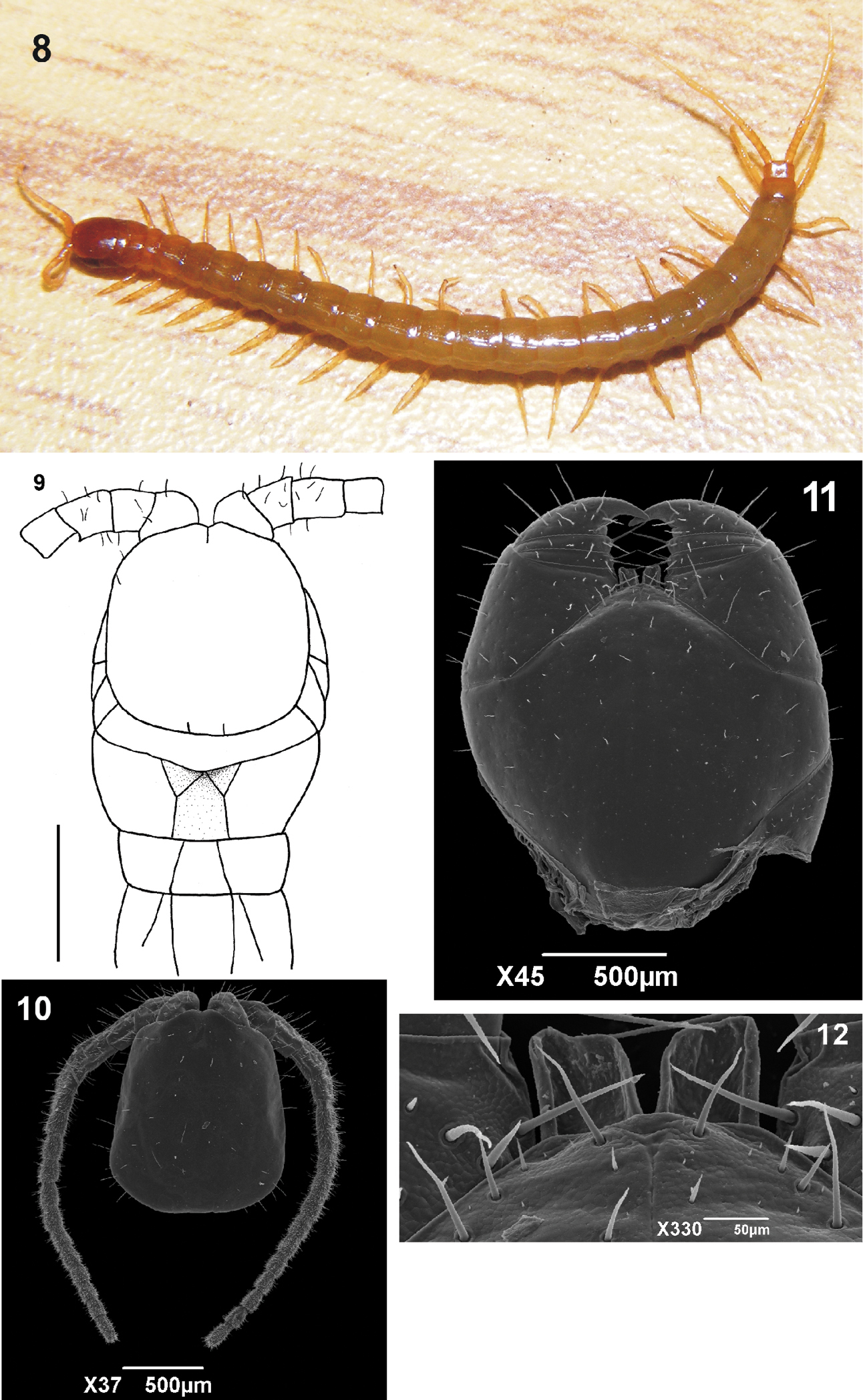
Body length: 22 to 28 mm (without ultimate legs); body in general yellowish (Figure 8). Cephalic plate and forcipular coxosternum brownish; antennae and legs yellowish. Cephalic plate smooth, with a short median suture on the anterior margin and a paired of short paramedian short sutures arising from posterior margin which overlies the first tergite (Figure 9).
Antennae composed of seventeen articles, first three (or four) articles glabrous, but with some long bristles, the succeeding ones becoming more densely clothed with fine short hairs (Figure 10).
Anterior margin of the forcipular coxosternum straight and narrow. The sides of the dentiform tooth plates are straight with the lateral sides longer than the medial sides; the anterior margins are concave (Figure 12). Forcipular coxosternum without sutures; none of the articles of forcipula armed; Tarsungula short and stout, apices alone overlapping in closed position (Figure 11).
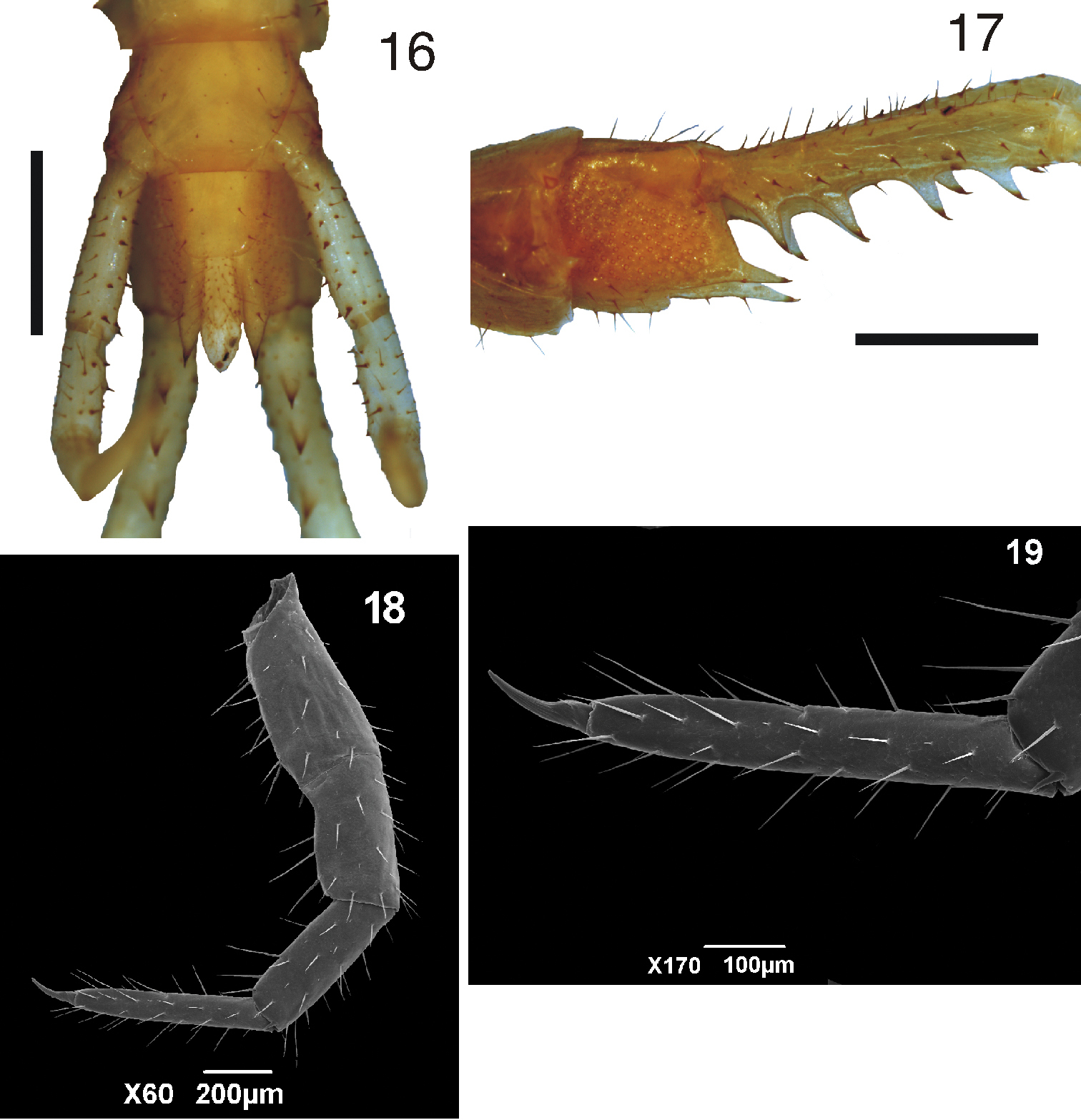
8 Tidops nisargani sp. n. Habitus 9 Tidops nisargani sp. n. Holotype (MNRJ 15350). Cephalic plate and the first three tergites. Dorsal view. Scale bar 1 mm 10 Tidops nisargani sp. n. (MNRJ). Cephalic plate showing the pilosity of the antennae. Dorsal view. Scale bar 50 um 11 Tidops nisargani sp. n. (MNRJ). Forcipules, showing the coxosternal tooth plates. Ventral view. Scale bar 500 um 12 Tidops nisargani sp. n. (MNRJ). Detail of the tooth plates. Ventral view. Scale bar 50 um.
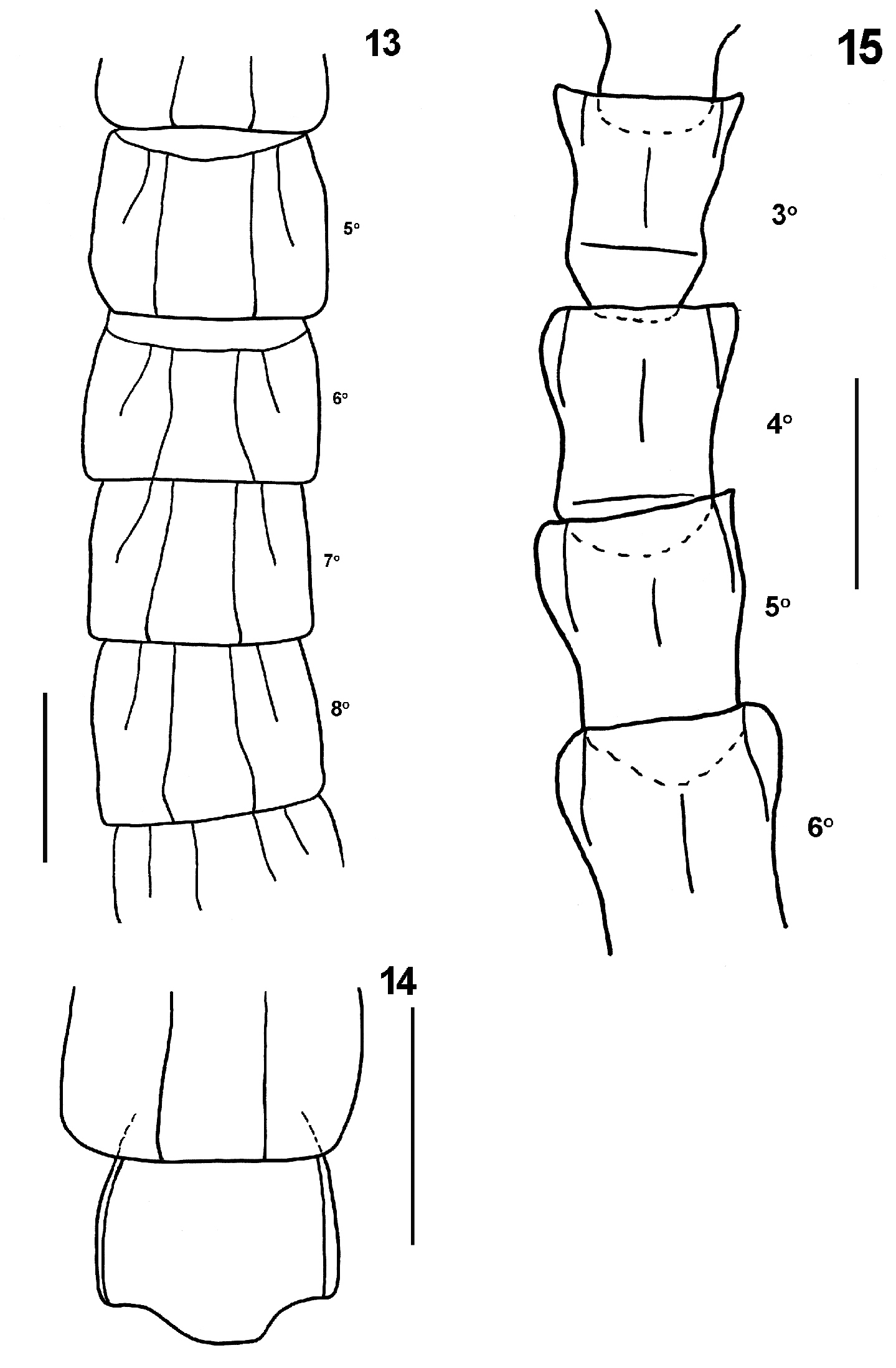
Tergites smooth; first tergite with a very distinct anterior transverse sulcus which strongly bends back in an angle at middle, to which is attached a median W-shape sulcus, from each of the posterior apices of which a pair of longitudinal sutures continue to the posterior margin of the tergite (Figure 9). There is a shallow depression in the middle of the anterior transverse sulcus and in the median W-shape. Tergites 2-22 with complete paramedian sutures and tergites 3-19 with lateral longitudinal sutures on the anterior two-thirds of each tergite (Figure 13). Only the 23rd tergite marginated. Margin of tergite 23 truncated medially and concave on each side, without a median sulcus, but with a shallow median depression (Figure 14).
Sternites smooth, longer than wide. With a large endosternite, well marked off by a transverse sulcus from the sternites 3-15, extending backwards under the sternites for one-third the length of the later (Figure 15). Sternites 3-21 with a median longitudinal sulcus; sternites 2-21 with two very distinct incomplete longitudinal lateral sutures separating off a narrow area at each side of the anterior portion of the sternites (Figure 15). Sternite 23 shorter than preceding, without sulci, lateral margin slightly convex and converging posteriorly; posterior margin straight, with corners rounded (Figure 16).
13 Tidops nisargani sp. n. Holotype (MNRJ 15350). Tergites 5 to 8 showing the paramedian and anterior lateral sutures. Dorsal view 14 Tidops nisargani sp. n. Holotype (MNRJ 15350). 23rd tergite 15 Tidops nisargani sp. n. Holotype (MNRJ 15350). Sternites 3 to 6. Scale bar 1 mm.
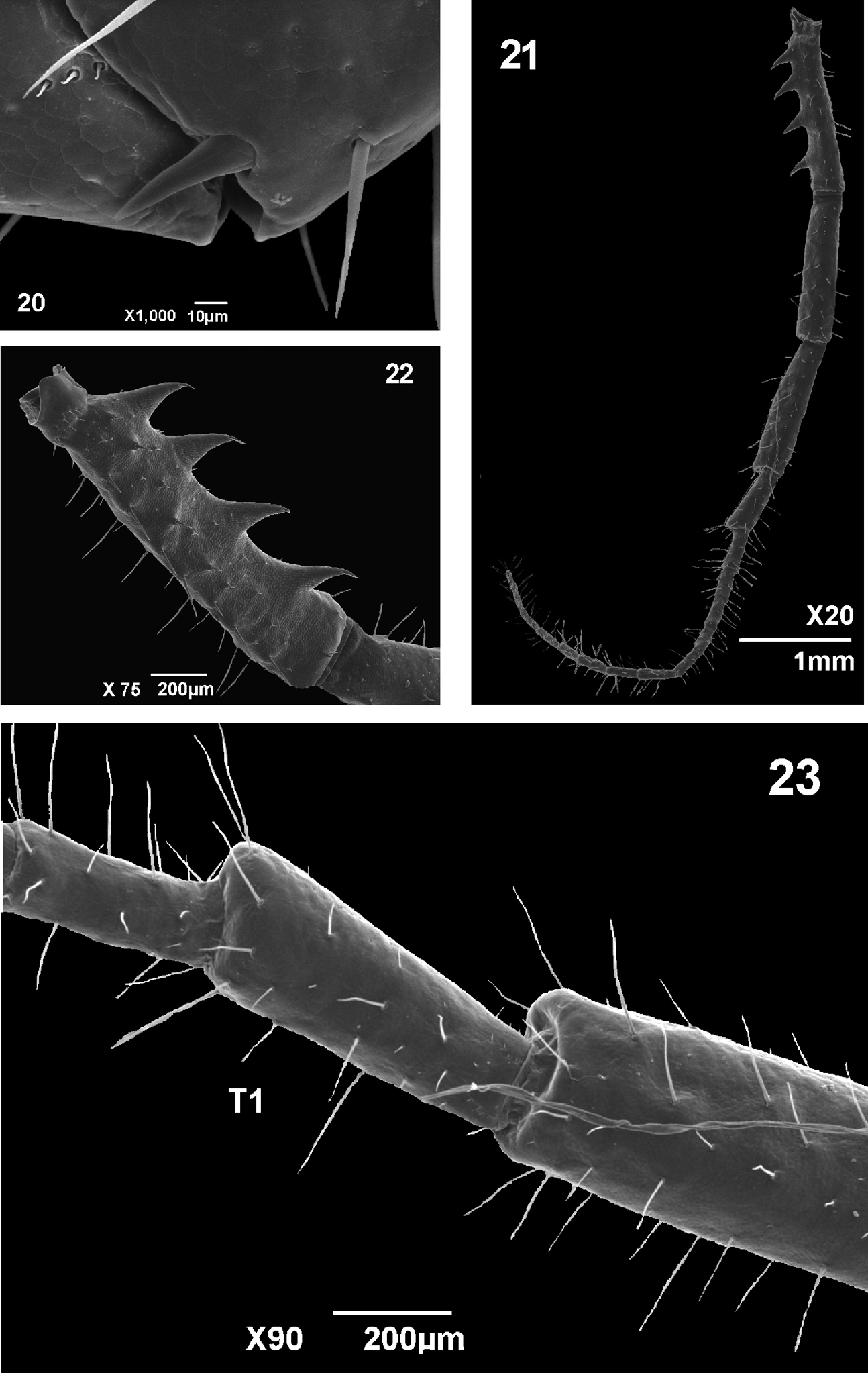
16 Tidops nisargani sp. n. Holotype (MNRJ 15350). Segments 22 and 23 showing 23rd sternite, ventral view 17 Tidops nisargani sp. n. Holotype (MNRJ 15350). Coxopleuron. Lateral view. Scale bar 1 mm 18 Tidops nisargani sp. n. (MNRJ). 8th locomotory leg. Lateral view. Scale bar 200 um 19 Tidops nisargani sp. n. (MNRJ). Detail of the tarsus 1 of the 8th locomotory leg. Lateral view. Scale bar 100 um.
Coxopleural process moderately long, with an acute apex (Figure 17). No lateral spines; dorsal and ventral surface of the coxopleuron bearing two long setae. Pores numerous, moderate-sized, evenly covering most of the surface of the coxopleuron (Figure 17).
Legs 1 to 21 with tarsi undivided (Figures 18 and 19), 22nd and 23rd divided. Tibia of the locomotory legs 3 to 20 with lateral distal spurs (holotype) (Figure 20), some specimen without. In all specimens from Amazonas and Pará the tibia of the locomotory legs lack a tibial spur. Ultimate legs slender and with many setae, articles usually longer than the articles of the ultimate legs of Tidops simus and Tidops balzanii (Figure 21); prefemur of the ultimate legs with a series of four large ventral processes (Figures 17 and 22) and a series of six or seven setae dorsomedially; femur and tibia without ventral or medial processes or spines, only with fine setae; tibia shorter than femur; femur and tibia without cylindric process at ventral corner of distal end; tarsus 1 short with a small expansion on the ventral corner of distal end (Figure 23); tarsus 2 divided into 9 to 15 distinct tarsomeres or “pseudoarticles”.
20 Tidops nisargani sp. n. Holotype (MNRJ). Detail of the tibial spine in the 8th locomotory leg. Lateral view. Scale bar 10 um 21 Tidops nisargani sp. n. Holotype (MNRJ). Ultimate leg. Lateral view. Scale bar 1 mm 22 Tidops nisargani sp. n. Holotype (MNRJ). Detail of the prefemur of the ultimate leg showing the ventral spinous processes. Lateral view. Scale bar 10 um 23 Tidops nisargani sp. n. Holotype (MNRJ). Detail of tarsus 1 of the ultimate leg showing the distal expansion. Lateral view. Scale bar 200 um.
Sítio Nisargan, Arraial d’Ajuda, Porto Seguro, state of Bahia, Brazil.
Brazil (Amazonas, Pará and Bahia).
Due to the similar structure of the ultimate legs of species of Tidops and Newportia these genera have been nearly always placed in a monophyletic clade, the Newportiinae.
Comparative data for Tidops simus, Tidops balzanii, Tidops collaris and Tidops nisargani sp. n.
| Characters | Species | |||
|---|---|---|---|---|
| Tidops simus | Tidops balzanii | Tidops collaris | Tidops nisargani | |
| Tooth plate | Longer than wide | Wider than long | Wider than long | Longer than wide |
| Distal margin of the tooth plate | Rounded | Rounded | Straight | Straight |
| Tibial lateral spurs | Present | Present | Present | Present or absent |
| Coxopleural process | Short | Short to medium | Medium to long | Medium to long |
| Prefemur of the ultimate legs | 3 ventral spinous processes | 3 ventral spinous processes | 4 ventral spinous processes | 4 (3) ventral spinous processes |
| Femur of the ultimate legs | 1 or 2 ventral spinous processes | without spinous processes | 2 ventral and 1 median spinous process | without spinous processes |
| Number of tarsomeres in tarsus 2 of the ultimate legs | 9 to 11 | 11 to 13 | 19 to 21 | 9 to 15 |
| Ventral cylindric process of the ultimate legs | On the femur, tibia and tarsus 1 | On the femur, tibia and tarsus 1 | Absent | Small distal expansion on tarsus 1 |
| Distribution | Grenada and Brazil | Brazil, Bolivia and Paraguay | Guyana, Venezuela, Brazil and Paraguay | Brazil |
The new species, Tidops nisargani, closely resembles Tidops collaris in the number of spinous ventral processes of the pre-femur of the ultimate pair of legs, but differs in the character of the femur. In the new species and in Tidops collaris, the pre-femur of the ultimate legs is armed with four large ventral spinous processes, but the new species differs from Tidops collaris in the absence of two small ventral and median single spinous process on the femur.
Specimens of Tidops nisargani from Amazonas and Pará also resemble Tidops balzanii. The pre-femur of the ultimate pair of legs is armed with three ventral spinous processes, the same number as seen in Tidops balzanii, however, these specimens differ from Tidops balzanii in the length of the tibia, the length of the coxopleural process and by the pilosity of the body and legs. In the new species the tibia is shorter than the femur, the coxopleural process is long to medium, and the body and legs are not very pilose whereas in Tidops balzanii the tibia is longer than or equal to the femur, the coxopleural process is medium to short, and the body and legs are more pilose than in the new species.
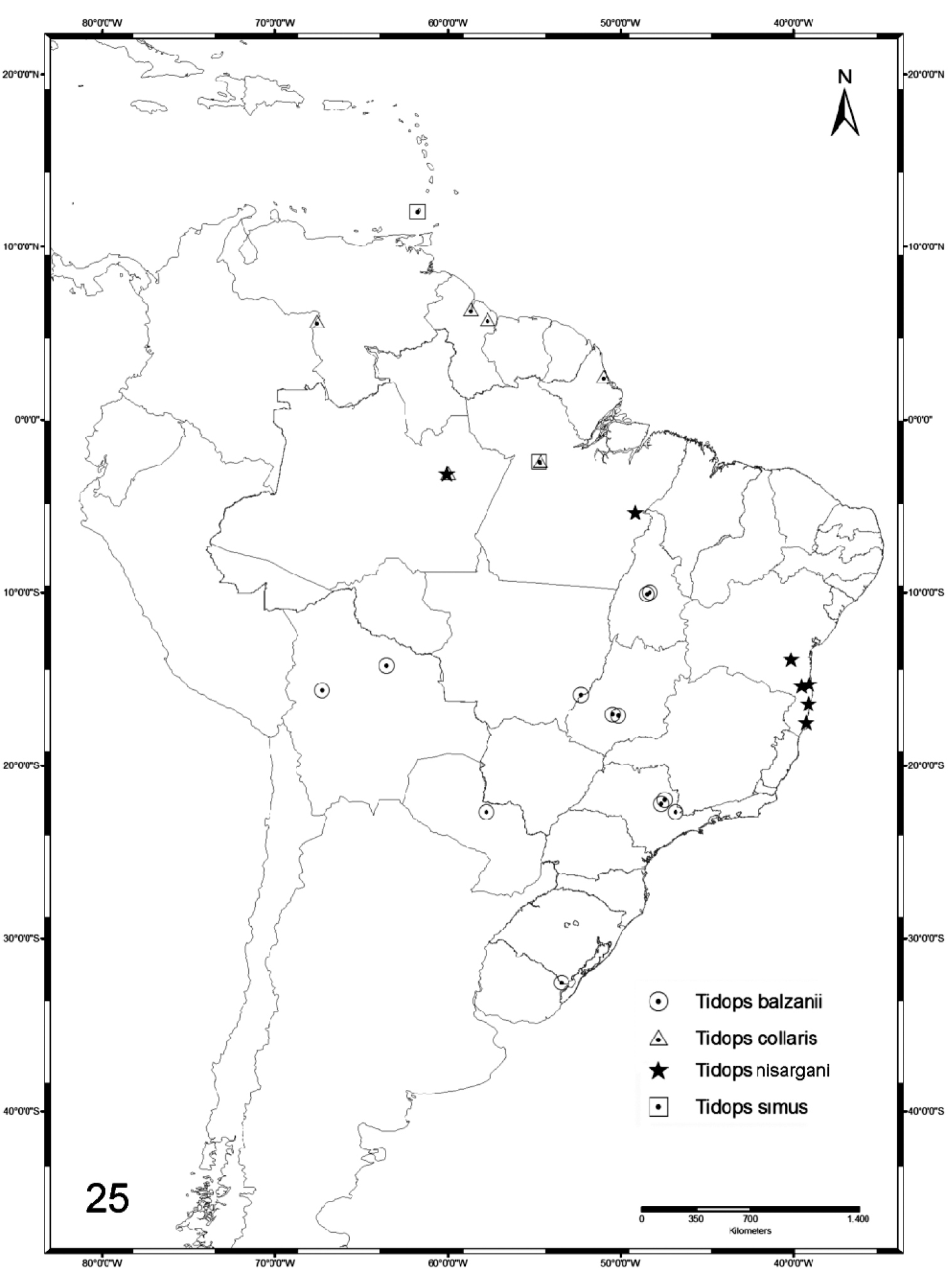
The genus Tidops occurs almost exclusively in South America, distributed from the northernmost country (Venezuela) to the extreme south of Brazil (Jaguarão, state of Rio Grande do Sul) (Figure 24). There is a single record of Tidops simus in the Lesser Antilles and no occurrence of the genus in Central America. The remaining species – Tidops simus, Tidops collaris and Tidops nisargani – occur further north in South America. Tidops simus, formerly known only from Grenada, but is here recorded from Santarém, state of Pará, Brazil. Tidops collaris is widely distributed in northern South America (Venezuela, Guyana and northern Brazil), except for one record of four specimens from San Lois in Paraguay published by
Map showing the distribution of the species of Tidops in the Neotropical Region.
Some historical records for Tidops collaris such as Carsevenne “Contesté Franco Brésilien” are now Brazil, state of Amapá. I found a specimen of Tidops balzanii from Rio Apa, Argentina. This specimen was probably collected by Capitano G. Bove or by L. Balzan in their travels to Argentina and Paraguay, respectively, or by Alfredo Borelli in his travels to Argentina and Paraguay. According
The genus Tidops is now composed of four species: Tidops simus, Tidops collaris, Tidops balzanii and Tidops nisargani sp. n. No specimens studied had a pair of spiracles on the seventh trunk segment. The ventral distal cylindric process present in the tibia of the ultimate pair of legs described for Tidops simus was also observed in Tidops balzanii as well as the distal enlargement of tarsus 1 of these species.
Kartops guianae, the only one species of the genus Kartops, was synonymized under Tidops collaris. Tidops is a genus typically from South America, with only one record for the Lesser Antilles. The four species of the genus occur in Brazil.
I am grateful to Hieronymus Dastych (ZMH), Jean-Jacques Geoffroy (MNHN), Janet Beccaloni (BMNH), Henrik Enghoff (ZMUC) and Gonzalo Giribet (MZC) for the facilities during my visit to these museums. To Greg Edgecombe (BMNH) to examine and take photos of the holotype of Kartops guianae and making available this essential key material for this study. To Elivaldo de Lima for SEM operation at the Center for Scanning Electron Microscopy of Museu Nacional, UFRJ. The establishment of this Center was made possible by a grant from Cenpes/Petrobras, and is part of the company’s Thematic Network for Marine Environmental Monitoring. This research was supported in part by Fundação de Amparo à Pesquisa do Estado de São Paulo (process 2010/10388-8). It was also supported in part by a “Mini-PEET” grant from the Society for Systematic Biology, an Ernst Mayr Grant, and a Collections Study Grant which funded travel by the author to the North Carolina Museum of Natural Sciences, Museum of Comparative Zoology and American Museum of Natural History, respectively. This research is also part of the Project “Aracnídeos e Miriápodes da Mata Atlântica” (AMMA), of the ARACNOLAB (Laboratory of arachnids of the Museu Nacional/UFRJ).