(C) 2011 I. I. Semenyuk. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Isotopic composition of nitrogen in 19 species of Diplopoda from a tropical monsoon forest (Cat Tien National Park, southern Vietnam) which supports one of the most diverse millipede faunules globally (no less than 36 species from 17 families and 11 orders) forms a wide continuum of δ15N values ranging from -2.4 to +6.8‰. This suggests a trophic niche differentiation among species. Variation in mouthpart structure could presumably reflect the different foods consumed by species representing at least higher taxa (families and orders). The fine structure of the mandibles in ten sympatric, mostly even syntopic species of Diplopoda does differ considerably between the higher taxa, but neither at the generic nor species level. Neither clear-cut trends in nor evident morphological patterns of, nor significant correlations between the structure of mandibles in Diplopoda species that have different isotopic compositions of nitrogen and presumably exploit different food resources, have been revealed.
Diplopoda, ecology, mandible, stable isotopes, seasonal tropical forest, Vietnam
Biological diversity of nearly all animal taxa, including Diplopoda, peaks towards the Equator. A range of hypotheses have been proposed to explain this phenomenon (
Ecological theory suggests that the maintenance of high species diversity levels requires ecological niche separation of different species (
In millipedes, the main physical processing of the ingested food items is believed to occur in the mouth cavity (
We presumed that variation in food types consumed by different species can be reflected in mouthpart structure. The aim of this study was to compare the mandibles of ten millipede species belonging to several higher taxa and differing in the isotopic composition of nitrogen in their tissues.
MethodsMillipedes and their potential food sources were collected in May–June 2008 in the Cat Tien National Park, Dong Nai Province, southern Vietnam (11°21'–11°48'N; 107°10'–107°34'E). The park covers an area of about 74, 000 ha, lying at the foot of the central Vietnamese highlands, about 130 km northeast of Ho Chi Minh City. The climate is tropical monsoon with two distinct seasons: a rainy season from late April to November and a dry season from December to March. The mean annual temperature is close to 26°C, with rather small seasonal fluctuations. The mean annual rainfall is about 2, 450 mm, the most rainy months being August and September (400–450 mm per month), when much of the park area is inundated. In contrast, there is almost no precipitation from January to March. The elevations vary between 120 and 220 m a.s.l. The relief is hilly with numerous small rocky outcrops and lowlands, the latter usually flooded for several weeks during the rainy season. Lowlands are especially characteristic of the eastern part of the area bordering the Dong Nai River. Sampling plots were selected in that area. Soils are mostly loamy, black ferrallitic, formed on basalt bedrock. The carbon content of the upper mineral soil typically varies from 3 to 8%; the pH is close to 5.5 in well-drained areas, but often decreases below 4.5 in wet depressions. Along the Dong Nai River, the soils are formed on sandy river sediments.
The vegetation of the National Park is very diverse and includes over 150 tree species, of which Lagerstroemia calyculata (Lythraceae) associated with Dipterocarpaceae, Fabaceae and Datiscaceae often dominates the upperstorey and canopy (
Diplopoda were collected by hand-sorting of soil and litter at five permanent plots (
Samples for isotopic analyses contained about 0.5 mg of dry animal tissue or 1.5–3 mg of dry plant or soil material. The isotopic composition of nitrogen (15N/14N) was determined with a Finnigan Delta V Plus isotope ratio mass spectrometer (Thermo, USA), coupled with a Flash 1112 elemental analyzer (Thermo, USA). Stable isotope abundance was expressed using the conventional δ notation with δ15N (‰) = [(Rsample – Rstandard)/Rstandard] × 1000, where Rsample and Rstandard represent the 15N/14N ratios of the sample and standard, respectively. Atmospheric N2 served as a primary standard. IAEA reference materials (USGS 40, USGS 41) were used for calibration. Acetanilide (Merck, Darmstadt) was used as a laboratory standard to correct drift (measured after every 10 samples). An analytical error of δ15N determination was less than ±0.2‰ (SD, eight repeated measurements of USGS 40). In total, 19 millipede species were analyzed.
Mandibles of ten species were extracted from alcohol-preserved animals, dried, mounted and examined using a Cam Scan MV2300 scanning electron microscope (Tescan, Brno).
ResultsThe Diplopoda community of Cat Tien consists of at least 36 species from 17 families and 11 orders (Table 1).
Taxonomic structure of the Cat Tien millipede community.
| 1. Alloproctoides aff. dawydoffi (Attems) | Family Lophoproctidae | Order Polyxenida |
| 2. Termitodesmus sp. | Family Termitodesmidae | Order Glomeridesmida |
| 3 & 4. Zephronia spp. 1 & 2 | ||
| 5. ?Prionobelum sp. | Family Zephroniidae | Order Sphaerotheriida |
| 6. Sphaerobelum sp. | ||
| 7. Hyleoglomeris sp. | Family Glomeridae | Order Glomerida |
| 8. Siphonophorella sp. | Family Siphonophoridae | Order Siphonophorida |
| 9. Pseudodesmus sp. | Family Andrognathidae | Order Platydesmida |
| 10. Metopidiothrix sp. | Family Metopidiotrichidae | Order Chordeumatida |
| 11. Thyropygus carli (Attems) | Family Harpagophoridae | Order Spirostreptida |
| 12. Plusioglyphiulus ampullifer Golovatch et al. | Family Cambalopsidae | |
| 13. Apeuthes sp. | Family Pachybolidae | Order Spirobolida |
| 14. Nepalmatoiulus sp. | Family Julidae | Order Julida |
| 15. Eutrichodesmus sp. | Family Haplodesmidae | |
| 16. Niponiella sp. | Family Cryptodesmidae | |
| 17. Cryptocorypha hoffmani Golovatch et al. | ||
| 18. Pseudocatapyrgodesmus pulcher Golovatch et al. | Family Pyrgodesmidae | |
| 19. Skotodesmus vietnamicus Golovatch et al. | ||
| 20. Desmoxytes pilosa (Attems) | ||
| 21. Desmoxytes cattienensis Nguyen et al. | ||
| 22–24. Orthomorpha spp. 1, 2, 3 | Order Polydesmida | |
| 25–28. “Tylopus” spp. 1, 2, 3, 4 | Family Paradoxosomatidae | |
| 29. Helicorthomorpha holstii (Pocock) | ||
| 30. Anoplodesmus anichkini Golovatch & Semenyuk | ||
| 31. Touranella cattiensis Golovatch & Semenyuk | ||
| 32. Nedyopus dawydoffiae (Attems) | ||
| 33–35. Unassigned spp. 1, 2, 3 | Family Fuhrmannodesmidae | |
| 36. “Martensodesmus” sp. | Family Opisotretidae |
Millipedes were found predominantly in leaf litter and at the soil/litter interface. Large-sized species, such as Thyropygus carli, Orthomorpha sp. 1, Sphaerobelum sp., Apeuthes sp., “Tylopus” sp. 1, Desmoxytes spp. and Helicorthomorpha holstii, were collected mainly from the litter surface. Smaller species like Alloproctoides aff. dawydoffi, Fuhrmannodesmidae spp. and “Martensodesmus” sp. occurred mostly in mineral soil, as did the juvenile Paradoxosomatidae. Sphaerotheriida species were largely absent from the soil samples, with only ?Prionobelum sp. collected from tree trunks and suspended soil having been analyzed.
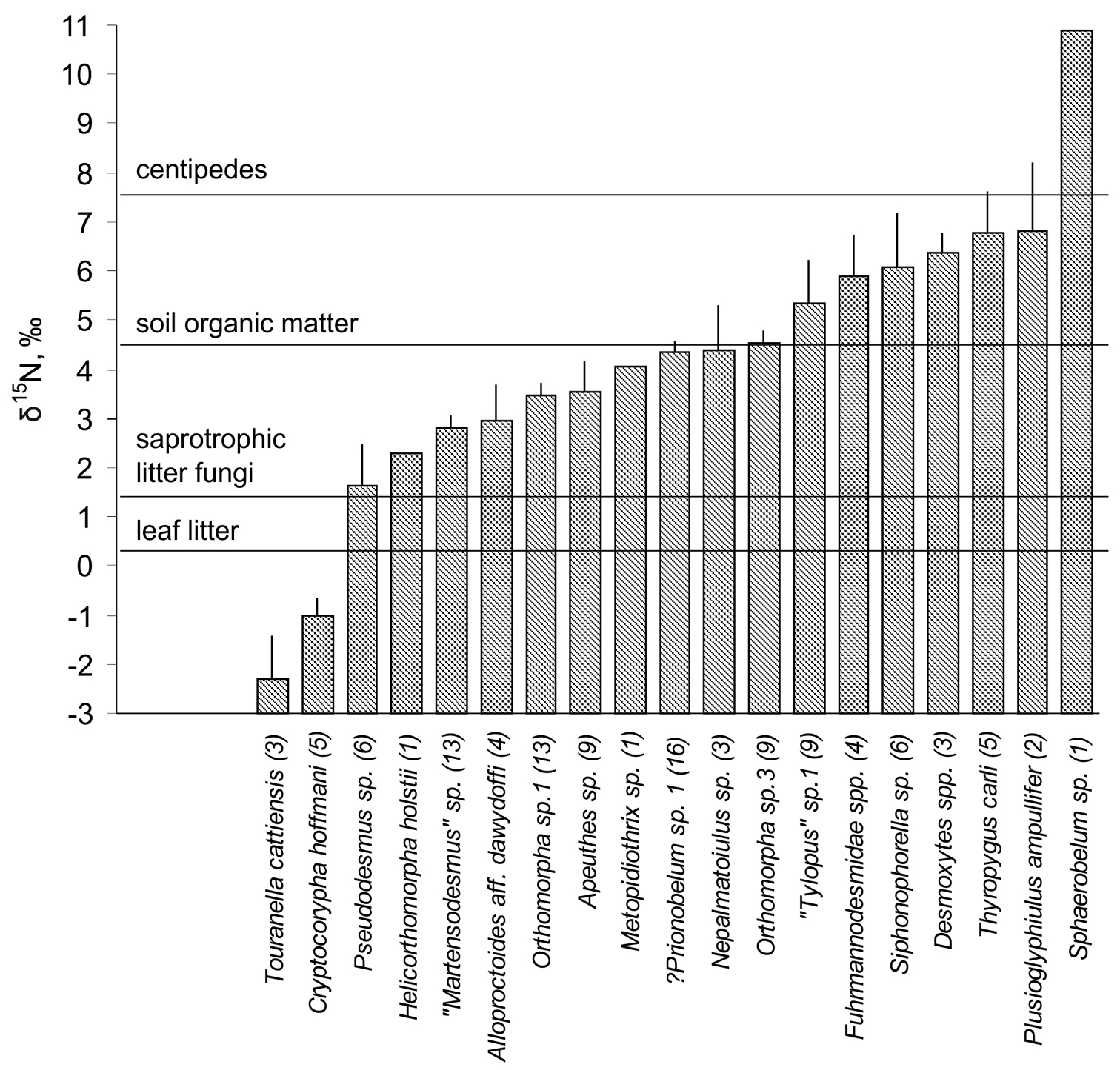
The isotopic composition of nitrogen (δ15N) of leaf litter, saprotrophic litter fungi and soil organic matter averaged 0.2±0.2‰, 1.5±0.3‰, and 4.5±0.5‰, respectively (Fig. 1). Most of the millipede species were enriched in 15N relative to leaf litter. However, the δ15N values of different Diplopoda varied widely and ranged from –2.3‰ (Touranella cattiensis) to +6.8‰ (Plusioglyphiulus ampullifer), the overall range of δ15N values exceeding 9‰ (Fig. 1). A similarly wide range of δ15N values, reflecting a diversity of trophic niches occupied, was revealed in other species-rich groups of litter-dwelling arthropods, e.g. collembolans (
Variation in δ15N values of 19 Diplopoda species from Cat Tien National Park (means + SE, number of specimens analysed is given in brackets). Horizontal lines show the mean δ15N values of leaf litter, saprotrophic litter fungi, soil organic matter and predatory centipedes.
The fine structure of the mandibles of the Diplopoda studied varied considerably between orders and, to a lesser extent, families (Table 2), i.e. well according to the known structural patterns (
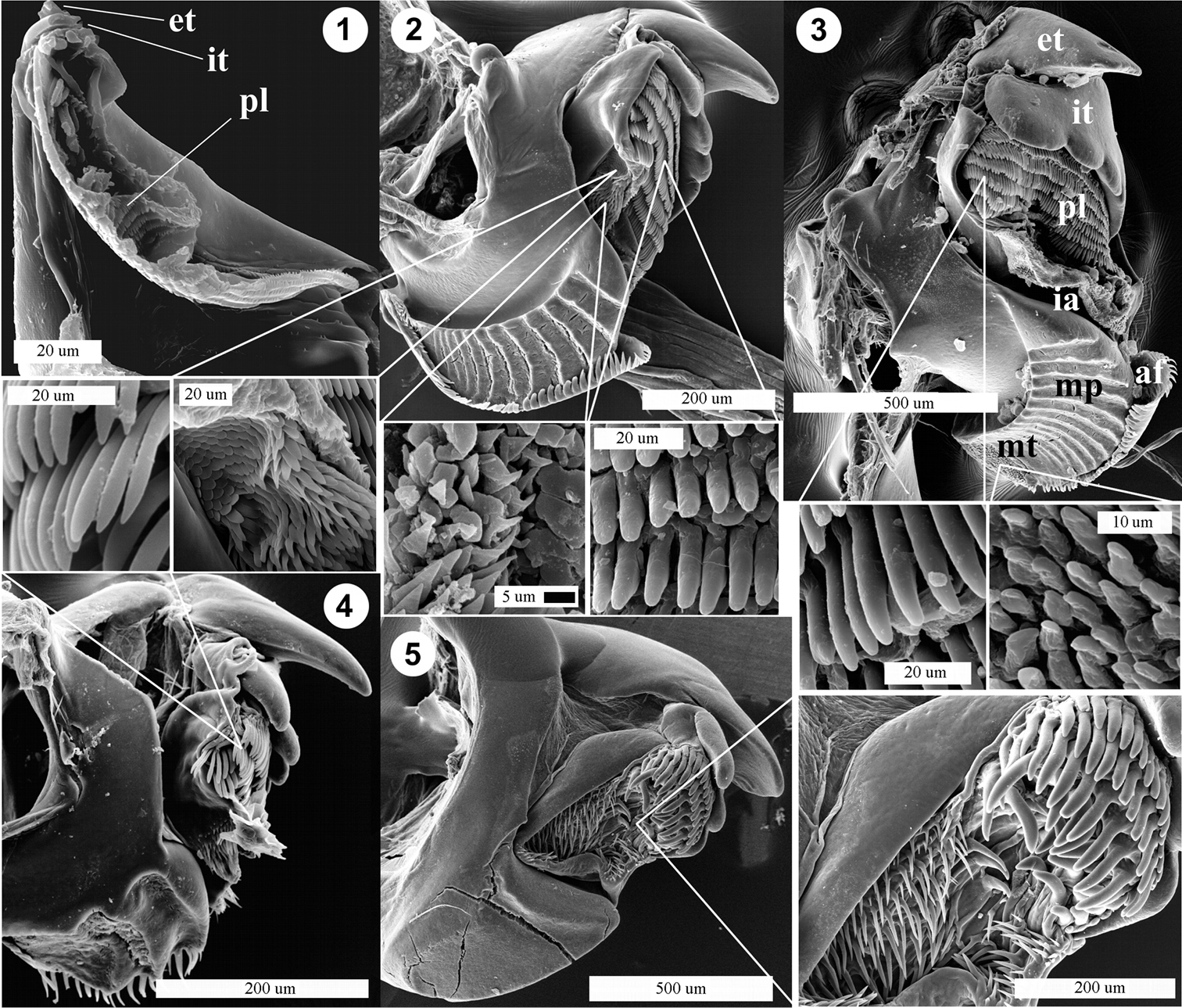
Mandibles of Diplopoda species with δ15N < 4.5‰. 1 Pseudodesmus sp. (abbreviations: et – external tooth, it – internal tooth, pl – pectinate lamellae) 2 Helicorthomorpha holstii (with two different parts of the intermediate area and pectinate lamellae teeth in a high-resolution inset) 3 Orthomorpha sp. 1 (with pectinate lamellae teeth and molar tufts, abbreviations: et – external tooth, it – internal tooth, pl – pectinate lamellae, ia – intermediate area, af – anterior fringe, mp – molar plate, mt – molar tuft) 4 Nepalmatoiulus sp. (with pectinate lamellae) 5 ?Prionobelum sp. (with pectinate lamellae teeth).
The mandible of all species studied shows well-developed external and internal teeth. The internal teeth, which are possibly used to separate food particles from the substrate, are somewhat better developed in the species having higher δ15N values (Fig. 3). The molar plates of various species show different types of surface, i.e. with dense stairs-like structures, or molar processes in terms of
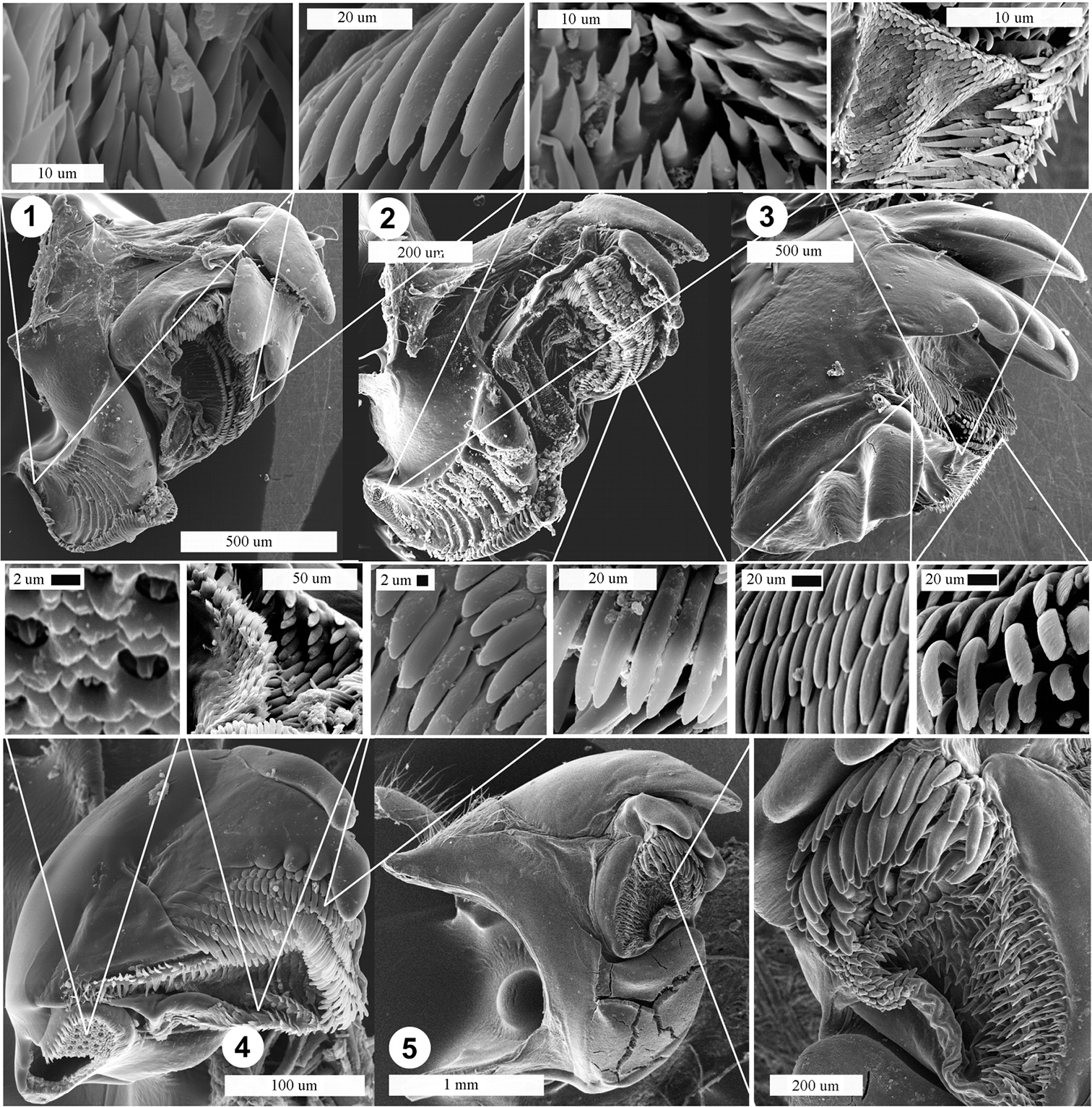
Mandibles of Diplopoda species with δ15N > 4.5‰. 1 “Tylopus” sp. 1 (with molar tufts and pectinate lamellae teeth high-zooming inset) 2 Desmoxytes sp. (with molar tufts and pectinate lamellae teeth) 3 Thyropygus carli (with part of intermediate area and two different kinds of pectinate lamellae teeth: straight conical and curved lamellar) 4 Plusioglyphiulus ampullifer (with tongue-liked structures on molar plate, intermediate area and teeth of pectinate lamellae) 5 Sphaerobelum sp. (with pectinate lamellae).
The mandibles of Plusioglyphiulus ampullifer differ significantly from those of the other species in showing a very small external tooth while the molar plates have unusual crater-like structures with tongues (Fig. 3: 4); these may have a sensory function. The pectinate lamellae are enlarged, which can indicate consumption of soft food or a filtering type of nutrition (
The δ15N values of animals are known to increase by 2–3.5‰ from one trophic level to the next one (
Among the species examined, all five basic morphotypes were present, i.e. polyxenoid, glomeroid, platydesmoid, polydesmoid and juloid (
The structure of the mouthparts of litter-dwelling millipedes seems to influence their assimilation rates. The density and relative size of pectinate lamellae teeth may correlate not only with the crushing efficiency of the mandibles, but also with body size (
The mandibles of the millipede orders have long been known to differ considerably in structure (
Generally speaking, the structure of millipede mandibles can well be presumed as being related to specializations for different kinds of food in species having different isotopic signatures. The fine structure of the mandibles of the Diplopoda studied here did vary considerably, especially well so between orders and, to a lesser extent, families (Figs 2 and 3; Table 2). Nevertheless, the variation in the mandibular structure of particular species having different δ15N values fails to support their separation into well-structured trophic guilds. The same seems true for the patterns of spatial distribution of species, both local and between-habitat.
Summarized characteristics of the mandibles and δ15N values in the studied Diplopoda species.
| Body length, mm | Number of internal teeth | Number of teeth rows on pectinate lamellae | Approx. density of teeth in pectinate lamellae | Character of molar plate | Presence of molar tuft at posterior end of molar plate | δ15N values, mean ± SE, ‰ | |
| Pseudodesmus sp. | 4–18 | - | 7 | 225 per 100 µm2 | - | - | 1.6±0.9 |
| Helicorthomorpha holstii | 4–30 | 4 | 6 | 6 per 100 µm2 | Stairs-like | Yes | 2.3 |
| Orthomorpha sp. 1 | 4–45 | 4 | 6 | 72 per 10, 000 µm2 | Stairs-like | Yes | 3.5±0.3 |
| Nepalmatoiulus sp. | 4–35 | 4 | 4 | 200 per 10, 000 µm2 | Large-scale stairs | ? | 4.4±0.9 |
| ?Prionobelum sp. | up to 15 | 3 | 7 | 15 per 10, 000 µm2 | Flat | No | 4.3±0.2 |
| “Tylopus” sp. 1 | 4–34 | 4 | 6 | 70 per 10, 000 µm2 | Stairs-like | Yes | 5.3±0.9 |
| Desmoxytes sp. | 5–18 | 4 | 6 | 95 per 10, 000 µm2 | Stairs-like | Yes | 6.4±0.4 |
| Thyropygus carli | 7–140 | 4 | 6 | 42 per 10, 000 µm2 | Large-scale stairs | No | 6.8±0.9 |
| Plusioglyphiulus ampullifer | 5–45 | 5 | 6 | 250 per 10, 000 µm2 | Notched | No | 6.8±1.4 |
| Sphaerobelum sp. | up to 43 | 3 | 5 | 6 per 10, 000 µm2 | Flat | No | 10.9 |
As a result, our studies show neither clear-cut trends in nor evident morphological patterns of, nor significant correlations between the structure of mandibles in Diplopoda species that have different isotopic compositions of nitrogen and presumably exploit different food resources. There is certain variation in mandibular structure of individual higher taxa, but in general the mouthparts are relatively uniform at the lower taxonomic levels. This is confirmed by both species of Zephroniidae (Sphaerotheriida), as well as in all Paradoxosomatidae (Polydesmida) under study.
We are most grateful to the Vietnamese-Russian Tropical Centre and the Administration of the Cat Tien National Park for the support and logistic help they rendered during our research in Vietnam. Special thanks go to the Organizing Committee of the 15th International Congress of Myriapodology, July 2011, Brisbane, Australia for supporting one of us (IIS) on the spot. The study was also supported by the Russian Foundation for Basic Research (grant No 11-04-00948).