(C) Henrik Enghoff. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Trans-segmental serial colour patterns, i.e., colour patterns consisting of repeated elements, each of which covers several diplosegments / body rings, are described from several millipede taxa: Centrobolus vastus (Attems, 1934) var. sexfasciatus Lawrence, 1967 (Spirobolida: Pachybolidae), Sagmatostreptus strongylopygus (Attems, 1950) (Spirostreptida, Spirostreptidae), and several groups of the order Platydesmida. The occurrence of similar patterns in Siphonocryptus zigzag Enghoff, 2010 (Siphonocryptida, Siphonocryptidae) and unidentified species of the order Chordeumatida is recorded. The patterns are shown in most cases to correlate with postembryonic growth, anamorphosis, i.e., each pattern elements corresponds to a set of diplosegments added during a moult.
Centrobolus, Sagmatostreptus, Platydesmus, Pseudodesmus, Brachycybe, Siphonocryptus, Australeuma, Schedotrigona, anamorphosis, aposematic
Millipedes have a general reputation of being discretely, not to say boringly coloured. There are, however, numerous exceptions, including such aptly named species as “the shocking pink dragon millipede” (
Millipedes may be uniformly coloured, or they may have more or less pronounced colour patterns. A very common pattern is one of transverse stripes, where each diplosegment is differently coloured on its front and hind parts. Instead of transverse stripes, there may be one or more contrastingly coloured spots on each diplosegment, or there may be one or more continuous longitudinal bands along the body.
Most millipedes have defensive glands secreting such repellant substances as hydrogen cyanide or benzoquinones (
Many millipede species have segmentally repeated colour patterns, e.g., contrastingly coloured paranota as in numerous species of Polydesmida. In some cases, the series is discontinous, for example in the European polydesmid Polydesmus collaris C.L. Koch. 1847, in which the paranota of body rings 4, 6, 8, 11, and 14 (i.e., the rings which do not carry ozopores) are contrastingly yellow on a dark brown background (
In a few cases, the colour pattern consists of repeated elements, each of which covers several diplosegments / body rings. These “trans-segmental serial colour patterns” are the subject of the present paper. The study was prompted by the discovery of Siphonocryptus zigzag Enghoff, 2010.
Material and methodsSpecimens were examined from the following collections: MRAC: Muséum Royal d’Afrique Centrale, Tervuren, Belgium. ZMUC: Natural History Museum of Denmark (Zoological Museum), University of Copenhagen, Denmark. Further information was extracted from images kindly provided by colleagues and/or found on the internet.
Interpretation of postembryonic development in Centrobolus and Sagmatostreptus is based on the ‘law of anamorphosis’ (
Several species of the South African genus Centrobolus (Spirobolida: Pachybolidae) are bright red in colour or have a striking red-black colour pattern. In one taxon, the colour pattern is of the trans-segmental type, i.e., Centrobolus vastus (Attems, 1934) var. sexfasciatus Lawrence, 1967 (Figs 1–2, see also
Centrobolus vastus var. sexfasciatus photographed by Guido Coza at Port. St. Johns, Eastern Cape, South Africa. See also http://www.flickr.com/photos/46608040@N04/5261677348/ (under Centrobolus fulgidus).
Centrobolus vastus var. sexfasciatus, female from Port. St. Johns, Eastern Cape, South Africa, date?, M. Boddely leg. (MRAC). The arrows point at the body rings which are the posteriormost podous rings in the inferred anamorphic stadia. N. Ioannou phot. Length of specimen 60 mm.
I have examined five males and five females collected at Port St. Johns, Eastern Cape, South Africa, date unknown, M. Boddely leg. (MRAC). This sample is very homogeneous: All specimens possess 43 podous body rings and have no apodous body rings in front of the telson, i.e., they have the body ring formula 43+0+T sensu
Functionally, this pattern can intuitively be interpreted as aposematic (
Like at least most other members of the order Spirobolida, Centrobolus species develop postembryonically through a process known as hemianamorphosis: During the first several moults (the anamorphic phase), new body rings and leg-pairs are added until a (still immature) stadium in which the adult number of body rings and leg-pairs is reached, whereafter further moults take place without the addition of body rings and leg-pairs (the epimorphic phase). During the anamorphic phase, there is a number of legless (apodous) body rings in front of the telson, and according to the so-called ‘rule of anamorphosis’, the legless body rings turn leg-bearing (podous) after the next moult. (The existence of mature specimens with apodous rings in a few spirobolidan species [
Anamorphic specimens of Centrobolus spp. recorded by
| Epimorphic | |||||||
|---|---|---|---|---|---|---|---|
| 6+10+T | 31+4+T → | 35+4+T → | 39+3+T → | 42+0+T | |||
| 6+12+T | 21+5+T → | 26+5+T → | 31+5+T → | 36+4+T → | 40+3+T → | 43+0+T | |
| 28+5+T | 37+4+T → | 41+3+T → | 44+0+T | ||||
| 23+6+T | 45+0+T | ||||||
| 20+5+T |
Numbers of apodous body rings in the last seven anamorphic stadia of pachybolid millipedes. Data from the compilation by
| Species | No. of podous rings in epimorphic spms | No. of apodous rings in last six anamorphic stadia | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| Dactylobolus bivirgatus (Karsch, 1881) | 35–39 | 4–5 | 4–5 | 4–5 | 4 | 3–4 | 2 | 1 |
| Pelmatojulus ligulatus (Voges, 1878) | 54–55 | 6 | 6 | 6 | 6 | 5 | 3 | 1 |
| Pelmatojulus insignis Saussure, 1860 | 53–56 | 5–6 | 5–6 | 5–6 | 5–6 | 4–5 | 3 | 1 |
| Epibolus pulchripes (Gerstäcker, 1873) | 51–54 | 6 | 6 | 6 | 6 | 5 | 3 | 2 |
| Crurifarcimen vagans Enghoff, 2011 | 56 | 6 | 6 | 6 | 6 | 5 | 3 | 3 |
The trans-segmental colour pattern in Centrobolus vastus var. sexfasciatus can thus be explained as a consequence of anamorphic development. This kind of pattern remains, as far as known, unique with the Spirobolida.
Sagmatostreptus strongylopygus (Attems, 1950) – a more complicated caseIn the east African spirostreptid Sagmatotreptus strongylopygus (Attems, 1950) the body is generally light brown, but in the central and posterior part a blackish pattern of 6–7 elements occurs (
Sagmatostreptus strongylopygus, subadult female from Amani, E Usambara Mts., Tanzania, 11 April 1985, T.G. Nielsen leg. (ZMUC), dorsal and lateral views. N. Ioannou phot. Length of specimen 117 mm.
Colour patterns in 26 specimens of Sagmatosreptus strongylopygus. “B” on a grey background indicates a clear black marking; “b” or “?” on a lighter grey background indicates indistinct and almost invisible markings; “a” on a yellow background indicates apodous body rings; “T” on a yellow background indicates the telson. No markings occur on body rings 1–20 on these specimens.
| Collection no. | Spm no. | Sex | Rings | Ø (mm) | RO | No. of podous rings | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 | 51 | 52 | 53 | |||||||
| ZMUC 00101372 | 1 | F | 53+0+T | 12, 4 | ? | B | B | B | B | B | B | B | b | b | T | |||||||||||||||||||||||||
| ZMUC 00101374 | 3 | F | 52+0+T | 8, 1 | ?13 | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | T | |||||||||||||||
| ZMUC 00101372 | 2 | jF | 53+0+T | 7, 2 | ?10 | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | T | ||||||||||||||||||
| ZMUC 00101496 | jF | 51+2+T | 6, 4 | 12! | B | B | B | B | B | B | B | B | B | B | B | b | B | B | B | B | B | B | B | a | a | T | ||||||||||||||
| ZMUC 00101337 | M | 52+T+0 | 10, 0 | ?16 | B | B | B | B | B | B | B | B | B | B | B | B | B | b | T | |||||||||||||||||||||
| ZMUC 00101509 | M | 52+0+T | 11, 0 | ? | B | B | B | B | B | B | B | B | B | B | B | B | B | B | T | |||||||||||||||||||||
| ZMUC 00101495 | M | 52+0+T | 10, 2 | ? | B | B | B | B | B | B | B | B | B | B | B | B | B | T | ||||||||||||||||||||||
| ZMUC 00101344 | F | 52+0+T | 11, 8 | ?16+ | b | b | b | b | b | T | ||||||||||||||||||||||||||||||
| ZMUC 00101374 | 1 | F | 52+0+T | 10, 8 | ?16 | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | T | ||||||||||||||||||
| ZMUC 00101374 | 2 | jF | 52+0+T | 7, 7 | ?15 | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | T | |||||||||||||||||
| ZMUC 00101372 | 3 | M | 52+0+T | 9, 4 | ? | b | b | b | b | b | b | b | b | b | b | b | T | |||||||||||||||||||||||
| ZMUC 00101372 | 4 | jF | 52+0+T | 8, 3 | ?14+ | B | B | B | B | B | B | B | B | B | B | B | B | B | T | |||||||||||||||||||||
| ZMUC 00101343 | jF | 49+3+T | 5, 2 | ?11 | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | a | a | a | T | ||||||||||||||
| ZMUC 00101338 | 1 | jF | 51+0+T | 7, 2 | ?14 | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | b | T | |||||||||||||||||
| ZMUC 00101338 | 2 | j?F | 51+0+T | 9, 5 | ?15 | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | T | ||||||||||||||||
| ZMUC 00101373 | M | 51+0+T | 9, 8 | ? | B | B | B | B | B | B | B | B | B | B | B | B | B | T | ||||||||||||||||||||||
| ZMUC 00101338 | 3 | M | 51+0+T | 9, 8 | ?16+ | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | b | b | T | |||||||||||||||||
| ZMUC 00101339 | F | 51+0+T | 11, 3 | ?17 | ? | B | B | B | B | B | B | B | B | B | B | ? | B | T | ||||||||||||||||||||||
| ZMUC 00101497 | 1 | jF | 51+0+T | 8, 1 | ? | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | T | ||||||||||||||||
| ZMUC 00101497 | 2 | jF | 50+1+T | 6, 1 | 12! | b | B | B | B | B | B | B | B | B | B | B | B | B | a | T | ||||||||||||||||||||
| ZMUC 00101493 | jM | 45+3+T | 4, 7 | 11 | B | B | b | B | B | B | B | B | B | B | B | B | B | a | a | a | T | |||||||||||||||||||
| ZMUC 00101340 | 1 | j | 34+5+T | 2, 5 | 5 | B | B | B | B | B | B | B | B | a | a | a | a | a | T | |||||||||||||||||||||
| ZMUC 00101341 | j | 34+5+T | 2, 5 | 5 | B | B | B | B | B | B | B | a | a | a | a | a | T | |||||||||||||||||||||||
| ZMUC 00101342 | 1 | j | 34+5+T | 2, 5 | 5 | B | B | B | B | B | B | a | a | a | a | a | T | |||||||||||||||||||||||
| ZMUC 00101340 | 2 | j | 33+5+T | 2, 5 | 5 | B | B | B | B | B | B | B | a | a | a | a | a | T | ||||||||||||||||||||||
| ZMUC 00101342 | 2 | j | 29+5+T | 1, 9 | 4 | B | B | B | B | a | a | a | a | a | T | |||||||||||||||||||||||||
The relatively few anamorphic specimens (e.g., Fig. 4) studied provide a clue to the significance of the patterns: The anamorphic specimen with 29 podous + 5 apodous body rings would, according to the ‘law of anamorphosis’ (cf. above under Centrobolus vastus var. sexfasciatus) give rise to a specimen with 34 podous rings. This specimen has black markings on rings 28–29, i.e., the two last podous rings, and just under half (11 out of 25) of the larger specimens have a series of black markings ending on ring 34. The anamorphic specimens with 33–34 podous + 5 apodous rings would give rise to individuals with 38–39 podous rings in the next stadium, and 16 out of 20 larger specimens have a series of black markings ending on ring 38 or 39. It thus seems that the posterior 1–4 out of a set of podous body rings acquired at a moult is distinguished by black markings. Using this ‘key’, the colour patterns can be translated into series of inferred body ring formulae for each specimen, see Table 4. In the table, the early stadia are denoted as “n RO” where RO stand for “rows of ocelli” – this is a common way to denote developmental stadia in millipedes because in many groups, one row of ocelli is added at each moult (
Sagmatostreptus strongylopygus, anamorphic juvenile from Amani, E Usambara Mts., Tanzania, 26 July 1974, I.B. Enghoff & H. Enghoff leg. (ZMUC), 34 podous + 5 apodous rings, 5 rows of ocelli. N. Akkari phot. Scale 2 mm.
Body ring formulae in 26 specimens of Sagmatosreptus strongylopygus. Formulae in bold on a yellow background were observed, other formulae were inferred from colour patterns.
| Collection no. | Spm no. | Sex | Body ring formulae | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3RO | 4RO | 5R0 | 5R0+1 | 5R0+2 | 5RO+3 | 5R0+4 | 5RO+5 | |||
| ZMUC 00101372 | 1 | F | 30+5+T | 35+5+T | 40+4+T | 44+4+T | 48+?+T | ? | 53+0+T | |
| ZMUC 00101374 | 3 | F | 24+5+T | 29+5+T | 34+5+T | 39+5+T | 44+4+T | 48+3+T | 51+?+T | 53+0+T |
| ZMUC 00101372 | 2 | jF | 25+5+T | 30+5+T | 35+4+T | 39+5+T | 44+4+T | 48+?+T | 53+0+T | |
| ZMUC 00101496 | jF | 24+6+T | 30+4+T | 34+5+T | 39+4+T | 43+4+T | 47+4+T | 51+2+T | 53+?0+T | |
| ZMUC 00101337 | M | 24+5+T | 29+5+T | 34+4+T | 38+5+T | 43+4+T | 47+3+T | 50+?+T | 52+0+T | |
| ZMUC 00101509 | M | 24+5+T | 29+5+T | 34+4+T | 38+5+T | 43+4+T | 47+?+T | ? | 52+0+T | |
| ZMUC 00101495 | M | 24+5+T | 29+5+T | 34+5+T | 39+4+T | 43+4+T | 47+?+T | ? | 52+0+T | |
| ZMUC 00101344 | F | 28+5+T | 33+5+T | 38+5+T | 43+4+T | 47+?+T | ? | 52+0+T | ||
| ZMUC 00101374 | 1 | F | 23+5+T | 28+5+T | 33+5+T | 38+5+T | 43+4+T | 47+?+T | ? | 52+0+T |
| ZMUC 00101374 | 2 | jF | 23+6+T | 29+4+T | 33+5+T | 38+4+T | 42+5+T | 47+3+T | 50+2?+T | 52+0+T |
| ZMUC 00101372 | 3 | M | 25+4+T | 29+6+T | 35+4+T | 39+4+T | 43+4+T | 47+?+T | ? | 52+0+T |
| ZMUC 00101372 | 4 | jF | 30+5+T | 35+5+T | 40+4+T | 44+4+T | 48+?+T | ? | 52+0+T | |
| ZMUC 00101343 | jF | 25+5+T | 30+5+T | 35+5+T | 40+5+T | 45+4+T | 49+3+T | 52+0?+T | ||
| ZMUC 00101339 | F | 24+5+T | 29+4+T | 33+5+T | 38+4+T | 42+4+T | 46+?+T | 51+0+T | ||
| ZMUC 00101338 | 1 | jF | 23+5+T | 28+5+T | 33+5+T | 38+4+T | 42+4+T | 46+3+T | 49+2?+T | 51+0+T |
| ZMUC 00101338 | 2 | j?F | 23+5+T | 28+5+T | 33+5+T | 38+4+T | 42+4+T | 46+4+T | 50+1?+T | 51+0+T |
| ZMUC 00101373 | M | 24+5+T | 29+4+T | 33+5+T | 38+4+T | 42+4+T | 46+?+T | 51+0+T | ||
| ZMUC 00101338 | 3 | M | 23+5+T | 28+5+T | 33+4+T | 37+5+T | 42+3+T | 45+3+T | 48+?+T | 51+0+T |
| ZMUC 00101497 | 1 | jF | 24+6+T | 30+4+T | 34+4+T | 38+4+T | 42+4+T | 46+3+T | 49+?+T | 51+0+T |
| ZMUC 00101497 | 2 | jF | 23+6+T | 29+4+T | 33+4+T | 37+5+T | 42+4+T | 46+?+T | 50+1+T | 51+0?+T |
| ZMUC 00101493 | jM | 23+5+T | 28+4+T | 32+5+T | 37+4+T | 41+4+T | 45+3+T | 48+?+T | ||
| ZMUC 00101340 | 1 | j | 24+5+T | 29+5+T | 34+5+T | 39+?+T | ||||
| ZMUC 00101341 | j | 24+5+T | 29+5+T | 34+5+T | 39+?+T | |||||
| ZMUC 00101342 | 1 | j | 24+5+T | 29+5+T | 34+5+T | 39+?+T | ||||
| ZMUC 00101340 | 2 | j | 24+4+T | 28+5+T | 33+5+T | 38+?+T | ||||
| ZMUC 00101342 | 2 | j | 23+6+T | 29+5+T | 34+?+T | |||||
The bewildering multitude of inferred and observed segment formulae is summarized in Table 5. The ranges of podous body rings in each inferred stadium are unoverlapping up to stadium 5RO+1.
Inferred and observed body ring formulae in Sagmatostreptus strongylopygus. The number of specimens with each formula is shown in parentheses after the formula, observed formulae indicated with underlined numbers. The last column shows the range of podous and apodous body rings inferrred/observed for each RO stadium.
| Stadium | Range | ||||||||
| 3 RO | 23+5+T (5) | 23+6+T (3) | 24+4+T (1) | 24+5+T (9) | 24+6+T (2) | 25+4+T (1) | 25+5+T (2) | 23-25+4-6 | |
| 4 RO | 28+4+T (1) | 28+5+T (6) | 29+4+T (4) | 29+5+T (7+1) | 29+6+T (1) | 30+4+T (2) | 30+5+T (4) | 28-30+4-6 | |
| 5 RO | 32+5+T (1) | 33+4+T (2) | 33+5+T (7+1) | 34+4+T (3) | 34+5+T (3+3) | 35+4+T (2) | 35+5+T (3) | 32-35+4-5 | |
| 5 RO+1 | 37+4+T (1) | 37+5+T (2) | 38+4+T (6) | 38+5+T (4) | 39+4+T (3) | 39+5+T (2) | 40+4+T (2) | 40+5+T (1) | 37-40+4-5 |
| 5 RO+2 | 41+4+T (1) | 42+3+T (1) | 42+4+T (6) | 42+5+T (1) | 43+4+T (7) | 44+4+T (4) | 45+4+T (1) | 41-45+3-5 | |
| 5 RO+3 | 45+3+T (1+1) | 46+3+T (2) | 46+4+T (1) | 47+3+T (2) | 47+4+T(1) | 48+3+T (1) | 49+3+T (1) | 45-49+3-4 | |
| 5 RO+4 | 50+1+T (1+1) | 51+2+T (1) | 50-51+1-2 | ||||||
| epimorphic spms | 51+0+T (6) | 52+0+T (8) | 53+0+T (3) | 51-53+0 |
Table 6 shows the most frequent inferred body ring increments during the transition from stadium n to stadium n+1.
Numbers of apodous body rings in specimens of Sagmatostreptus strongylopygus in which at least 4 successive numbers could be inferred or observed.
| Collection no. | Spm no. | 3RO | 4RO | 5RO | 5RO+1 | 5RO+2 | 5RO+3 | 5RO+4 | Unimodality |
|---|---|---|---|---|---|---|---|---|---|
| ZMUC 00101372 | 1 | 5 | 5 | 4 | 4 | yes | |||
| ZMUC 00101374 | 3 | 5 | 5 | 5 | 5 | 4 | 3 | yes | |
| ZMUC 00101372 | 2 | 5 | 5 | 4 | 5 | 4 | NO | ||
| ZMUC 00101496 | 6 | 4 | 5 | 4 | 4 | 4 | NO | ||
| ZMUC 00101337 | 5 | 5 | 4 | 5 | 4 | 3 | NO | ||
| ZMUC 00101509 | 5 | 5 | 4 | 5 | 4 | NO | |||
| ZMUC 00101495 | 5 | 5 | 5 | 4 | 4 | yes | |||
| ZMUC 00101344 | 5 | 5 | 5 | 4 | yes | ||||
| ZMUC 00101374 | 1 | 5 | 5 | 5 | 5 | 4 | yes | ||
| ZMUC 00101374 | 2 | 6 | 4 | 5 | 4 | 5 | 3 | NO | |
| ZMUC 00101372 | 3 | 4 | 6 | 4 | 4 | 4 | yes | ||
| ZMUC 00101372 | 4 | 5 | 5 | 4 | 4 | yes | |||
| ZMUC 00101343 | 5 | 5 | 5 | 5 | 4 | 3 | yes | ||
| ZMUC 00101339 | 5 | 4 | 5 | 4 | 4 | NO | |||
| ZMUC 00101338 | 1 | 5 | 5 | 5 | 4 | 4 | 3 | yes | |
| ZMUC 00101338 | 2 | 5 | 5 | 5 | 4 | 4 | 4 | yes | |
| ZMUC 00101373 | 5 | 4 | 5 | 4 | 4 | NO | |||
| ZMUC 00101338 | 3 | 5 | 5 | 4 | 5 | 3 | 3 | NO | |
| ZMUC 00101497 | 1 | 6 | 4 | 4 | 4 | 4 | 3 | yes | |
| ZMUC 00101497 | 2 | 6 | 4 | 4 | 5 | 4 | 1 | NO | |
| ZMUC 00101493 | 5 | 4 | 5 | 4 | 4 | 3 | NO |
Looking at the complete sequences, there are hardly two specimens with identical inferred sequences, although some come close:
23+5+T → 28+5+T → 33+5+T → 38+4+T → 42+4+T → 46+3–4+T: specimens ZMUC 00101338(1) and ZMUC 00101338(2), which have the epimorphic formula 51+0+T
23+5+T → 28+5+T → 33+5+T → 38+5+T → 43+4+T → 47+3/?+T. This pathway is followed by specimens ZMUC 00101344 (stadium 3RO missing) and ZMUC 00101374(1) which have the epimorphic formula 52+0+T
The inferrred course of anamorphosis in Sagmatostreptus strongylopygus is consistent with that found in another spirostreptid, Archispirostreptus tumuliporus (Karsch, 1881) (= Graphidostreptus tumuliporus) (
In the course of anamorphosis of juliformian millipedes, the number of apodous rings usually reaches a maximum early in development and thereafter decreases monotonically (reaching 0 in hemianamorphic species like Sagmatostreptus strongylopygus), i.e., a unimodal distribution of numbers of apodous rings over developmental time (
Deviations from the unimodal pattern have been observed in a few other millipede species belonging to the family Julidae (order Julida) (
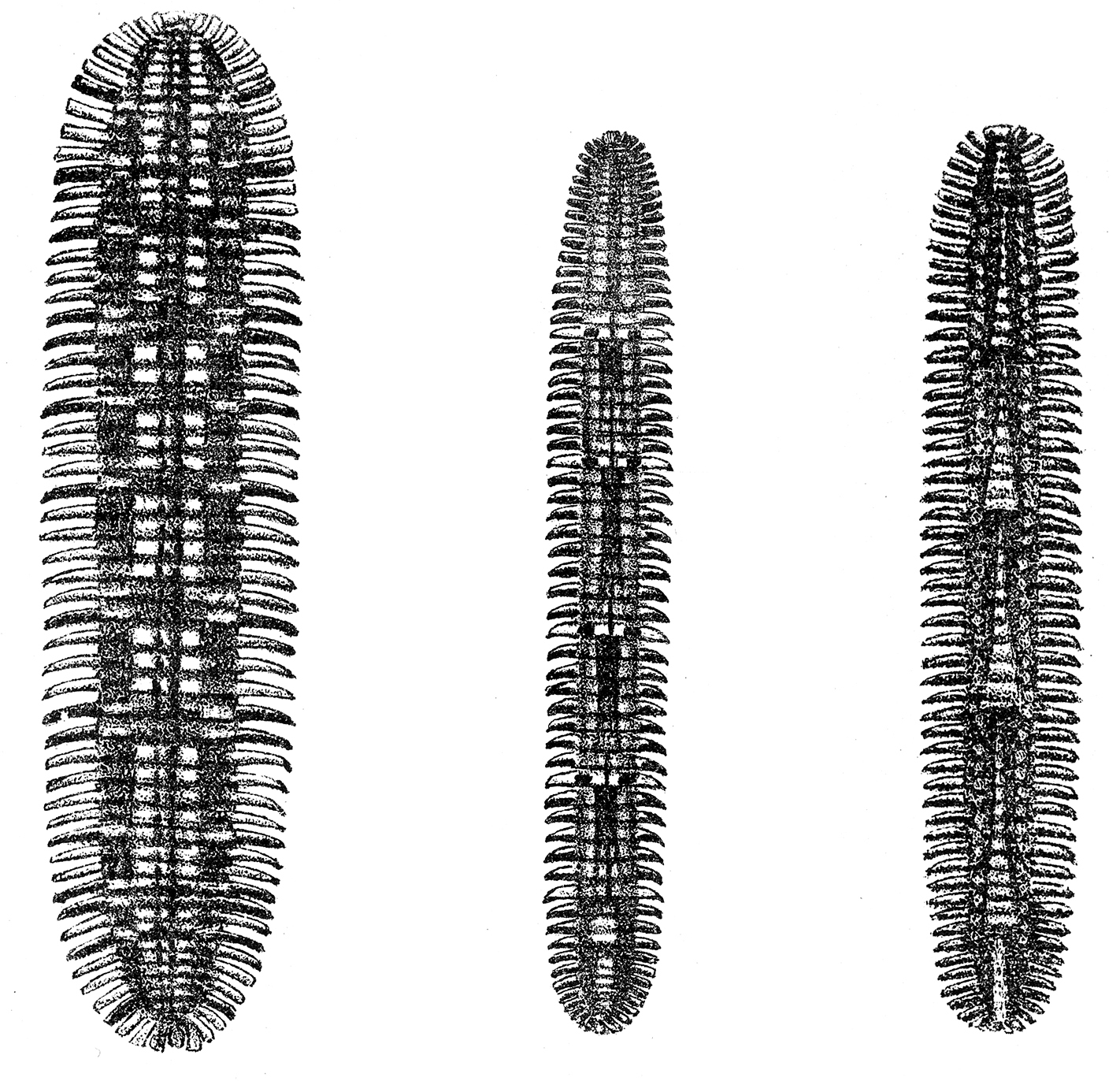
Quite complicated and irregular trans-segmental colour patterns occur in several species of the order Platydesmida. For example,
Left: Platydesmus perpictus Pocock, 1910, Middle: Platydesmus analis Pocock, 1910, Right: Platydesmus triangulifer Pocock, 1910. From
Platydesmus perpictus Pocock, 1910, has about seven multi-pleurotergite pattern elements, with limits between elements at pleurotergites 15, 23, 27, 32, 37, 42, 45 and 47 (as far as can be judged from the illustration, Fig. 5A, the same reservation applies to the following two species).
Platydesmus analis Pocock, 1910, has six multi-pleurotergite pattern elements. For this species,
Platydesmus triangulifer Pocock, 1910, has six multi-pleurotergite pattern elements, according to
The patterns in these Platydesmus species are summarised in Table 7.
Transsegmental colour patterns in Platydesmus species described by
| A: | ||||||||||
| Platydesmus perpictus | 15 | 23 | 27 | 32 | 37 | 42 | 45 | 47 | ||
| Platydesmus analis | 14 | 22 | 32 | 40 | 46 | 50 | ||||
| Platydesmus triangulifer | 6 | 15 | 24 | 34 | 45 | 52 | ||||
| B: | ||||||||||
| Platydesmus perpictus | 8 | 4 | 5 | 5 | 5 | 3 | 2 | |||
| Platydesmus analis | 8 | 10 | 8 | 6 | 4 | |||||
| Platydesmus triangulifer | 9 | 9 | 10 | 11 | 7 | |||||
Thanks to Paul Marek, I have become aware of a striking photo by Steve Taylor, featuring a group of platydesmids from Costa Rica (Fig. 6, the photo is also available at http://www.flickr.com/photos/ceuthophilus/147312880.
Unidentified platydesmids from Costa Rica, P. Marek & J.E. Bond leg. Adult specimens code: Left top: 1 left bottom 2 middle top 3 middle middle 4 middle bottom 5 right top 6 right middle 7 right bottom 8 cf. text and table 8 S. Taylor phot. See also http://www.flickr.com/photos/ceuthophilus/147312880.
There are 8 adult individuals on the photo. Each has 4–6 transsegmental pattern elements. Each element consists of a triangular light marking on each side of the midline, each triangle covering 3–5 pleurotergites and becoming narrower at its posterior end. The elements are separated by 2–3 unmarked pleurotergites. The anterior limit of each element is sharply marked. The anterior end of the specimens is difficult to identify on most specimens, so the absolute tergum numbers may be wrong by one or two. This is, however, less important – the important thing is the nature of the periodicity. Table 8 summarises the patterns in these specimens.
Beautiful photos of a Platydesmus sp. from Guatemala are available at http://www.nadiplochilo.com/oplatydesmida.html but the colour pattern could not be analysed.
Trans-segmental colour patterns in unidentified platydesmid from Costa Rica (Fig. 5). A: Entries are limits between pattern elements, given as the number of the anteriormost pleurotergite in each pattern element. An attempt has been made to place pleurotergite numbers corresponding to the same stadium in the same column. B: entries are differences, expressed as no. of pleurotergites, between one limit and the one in front of it or, in other words, numbers of pleurotergites constituting one colour pattern element.
| A: | ||||||||
| specimen 1 | 12 | 19 | 26 | 33 | ||||
| specimen 2 | 8 | 16 | 21 | 29 | ||||
| specimen 3 | 14 | 21 | 29 | 37 | ||||
| specimen 4 | 14 | 20 | 27 | 33 | ||||
| specimen 5 | 14 | 20 | 25 | 31 | 37 | 42 | ||
| specimen 6 | 7 | 15 | 20 | 26 | 33 | |||
| specimen 7 | 7 | 14 | 21 | 28 | 35 | |||
| specimen 8 | 7 | 15 | 22 | 28 | 35 | 40 | ||
| B: | ||||||||
| specimen 1 | 7 | 7 | 7 | |||||
| specimen 2 | 8 | 5 | 8 | |||||
| specimen 3 | 7 | 8 | ||||||
| specimen 4 | 6 | 7 | 6 | |||||
| specimen 5 | 6 | 5 | 6 | 6 | 5 | |||
| specimen 6 | 8 | 5 | 6 | 7 | ||||
| specimen 7 | 7 | 7 | 7 | 7 | ||||
| specimen 8 | 8 | 7 | 6 | 7 | 5 | |||
Two variegated species of Andrognathidae have been described from Vietnam and Laos: Sumatronium variegatum Attems, 1938b, and Sumatronium persimile Attems, 1953. Generic concepts in SE Asian andrognathids are not at all settled and are subject of ongoing research by Peter Decker – for the time being I will refer all concerned species to the genus Pseudodesmus (the oldest available genus name based on a SE Asian species of this group). Recently, a specimen with a similar colour pattern has been photographed in Malaysia by P. Pimvichai (Fig. 7).
Unidentified platydesmid from Malaysia. P. Pimvichai phot.
I have examined a sample of Pseudodesmus cf. variegatus from Laos. The dorsal colour pattern of adults is highly complicated and irregular (Fig. 8). The general background is greyish brown, but is interrupted by four types of coloured components:
Pseudodesmus cf. variegatus from LAOS, Vientiane Prov., Phou Khao Khouay, N 18, 20, 369', E 102, 48, 523', 7–800 m, 28–31.v.2008, A. Solodovnikov & J. Pedersen leg. ( ZMUC), posterior part of body. Length of fragment 23 mm.
Paratergal spots: clear lateral yellowish spots which occupy the middle of a paratergite (lateral ‘wing’). These spots occur in groups, and the posteriormost spot in each group is usually enlarged and clearly marks the limit of the pattern element. (This is in contrast to the patterns in Platydesmus where the anterior limit of the pattern elements is clearly demarcated.)
Less well-defined yellowish brown spots which occupy an area roughly corresponding to the basal third of the paratergites and is delimited vis-a-vis the lateral spots and vis-a-vis the paramedian tubercles by a small blackish area. These spots occur on all pleurotergites.
Pale tubercular spots: clear paramedian yellowish spots which occupy the two consecutive paramedian tubercles on a pleurotergite. These spots occur in an irregular pattern along the body, interrupted by the belowmentioned black spots.
Dark tubercular spots: paramedian black spots which occupy the two consecutive paramedian tubercles on a pleurotergite.
Paratergal and tubercular spots show asymmetrical patterns, see, e.g., Table 9. Analysis of the pattern shown in Table 9 reveals, however, that in spite of the apparent chaos there are some regularities:
Occurrence of lateral and paramedian colour components along the body of a Pseudodesmus cf. variegatus female with 65 pleurotergites (excluding telson) ZMUC 00101498/A. Occurrence is indicated by ”+” for the lateral component (yellowish spot on paratergum), and by black and white squares (□ and ■, for pale and dark spots, respectively) for the paramedian tubercular spots. ++ indicates the posterior, enlarged lateral spot in a group.
| Lateral left | Paramedian tubercle left | Paramedian tubercle right | Lateral right | |
|---|---|---|---|---|
| Tergite no. | ||||
| 1 | ||||
| 2 | ||||
| 3 | ||||
| 4 | ||||
| 5 | ||||
| 6 | □ | □ | ||
| 7 | □ | ■ | ||
| 8 | ■ | □ | ||
| 9 | □ | ■ | ||
| 10 | ■ | □ | ||
| 11 | □ | ■ | ||
| 12 | ■ | □ | + | |
| 13 | □ | □ | ||
| 14 | + | □ | □ | + |
| 15 | ■ | □ | ||
| 16 | ■ | □ | ||
| 17 | ■ | □ | + | |
| 18 | ■ | □ | ||
| 19 | + | □ | ■ | |
| 20 | ■ | □ | + | |
| 21 | + | □ | □ | + |
| 22 | + | □ | □ | ++ |
| 23 | ■ | □ | ||
| 24 | + | □ | ■ | |
| 25 | + | □ | ■ | |
| 26 | + | □ | ■ | |
| 27 | □ | □ | + | |
| 28 | ++ | □ | □ | ++ |
| 29 | ■ | ■ | ||
| 30 | ■ | ■ | ||
| 31 | □ | ■ | ||
| 32 | + | □ | ■ | |
| 33 | ■ | □ | + | |
| 34 | + | □ | □ | + |
| 35 | + | □ | ■ | |
| 36 | + | □ | □ | + |
| 37 | ++ | □ | □ | ++ |
| 38 | ■ | ■ | ||
| 39 | ■ | □ | + | |
| 40 | + | □ | ■ | |
| 41 | + | □ | □ | + |
| 42 | + | □ | □ | + |
| 43 | + | □ | □ | + |
| 44 | ++ | □ | □ | ++ |
| 45 | + | □ | ■ | |
| 46 | + | □ | □ | + |
| 47 | ++ | □ | □ | ++ |
| 48 | ■ | ■ | ||
| 49 | ■ | □ | ||
| 50 | + | □ | ■ | + |
| 51 | + | □ | ■ | |
| 52 | + | □ | □ | |
| 53 | + | □ | □ | + |
| 54 | ++ | □ | ■ | |
| 55 | ■ | □ | ||
| 56 | + | □ | ■ | |
| 57 | + | □ | □ | + |
| 58 | + | □ | □ | + |
| 59 | ■ | ■ | ||
| 60 | ■ | □ | ||
| 61 | + | □ | □ | + |
| 62 | ||||
| 63 | ||||
| 64 | ||||
| 65 |
There is a strong tendency for the often enlarged posteriormost lateral spot in a group to occur on both sides of the same pleurotergite, in casu pleurotergites nos. (14), 22, 28, 37, 44, 47, [54], 58, (61) where numbers in round parentheses indicate ’groups’ of one spot, and the number in square brackets indicates asymmetry.
Pale tubercular spots (72 instances) are more frequent than dark ones (40 instances).
Pleurotergites with two dark tubercular spots are infrequent: 4 instances, compared with 21 instances of double-white and 31 instances of mixed colours.
There is a highly significant correlation (P= 5, 33086E-13, chi2-test, 2×2 matrix, Table 10) between presence of a lateral spot and the colour of the tubercular spot on the same side of the same pleurotergite.
Correlation between colour of tubercular spots and presence of lateral spots in a Pseudodesmus cf. variegatus female (ZMUC 00101498/A).
| Tubercular spot white | Tubercular spot black | |
|---|---|---|
| Lateral spot present | 53 | 1 |
| Lateral spot absent | 19 | 39 |
(Follows almost automatically from previous): There is a tendency for the tubercular sports to be pale on both sides on the posteriormost pleurotergite of each lateral group. Thus, both tubercular spots are pale on pleurotergites 14, 22, 28, 37, 44, 47, 58 and 61, i.e. on all the pleurotergites mentioned in the previous statement except the dubious pleurotergite 54. The preponderance is highly significant (P ~0.0005, chi2-test, 2×2 matrix).
Table 11 shows colour pattern statistics for four specimens. It is seen that the pattern regularities found in the specimens tabulated in Table 9 are quite general although the female with 63 pleurotergirtes has more black tubercular spots than the others. Table 12 shows the position of the limits between pattern elements (= position of enlarged posteriormost lateral spots in a group) in all ten specimens in the sample, and the distance (no. of pleurotergites) between element limits. On specimens with < 55 pleurotergites, which are generally much paler, no lateral spots are visible, but the irregular distribution of black and white tubercular spots is clear. Limits between pleurotergite series on these specimens were identified by locating the posterior limit of (series of) double white tubercular spots.
Colour pattern statistics for four specimens of Pseudodesmus cf. variegatus.
| ♀ 65 pleurotergites | ♂ 63 pleurotergites | ♀ 63 pleurotergites | ♂ 56 pleurotergites | |
|---|---|---|---|---|
| Pattern on tergites | 6–61 | 7–59 | 6–61 | 6–50 |
| White/black tubercular spots | 72/40 | 62/44 | 55/57 | 54/36 |
| Double-black/double-white/mixed pair of tubercular spots | 4/21/31 | 4/21/28 | 15/13/28 | 8/17/20 |
| White/black tubercular spot on hemitergite with lateral spot | 53/1 | 47/0 | 41/1 | 37/0 |
| White/black tubercular spot on hemitergite without lateral spot | 19/39 | 15/44 | 14/56 | 17/36 |
| P (chi2, 2×2 matrix) for values in rows 4 and 5 | 5, 33086E-13 | 9, 85096E-15 | 1, 79343E-15 | 9, 67137E-11 |
Position of enlarged posteriormost lateral spots in a group of spots in ten specimens of Pseudodesmus cf. variegatus ZMUC 00101498. (14), 22, 28, 37, 44, 47, [54], 58, (61) where symbols in round parentheses indicate ’groups’ of one spot, symbol in square brackets indicates asymmetry (spot only present on one side).
| A: | |||||||||
| ♀ 65 tergites | (14) | 22 | 28 | 37 | 44 | 47 | [54] | 58 | (61) |
| ♂ 63 tergites | 19 | 26 | 34 | 42 | 49 | 55 | 59 | ||
| ♀ 63 tergites | (12) | 18 | 25 | 33 | 41 | 48 | 54 | 58 | (61) |
| ♂ 56 tergites | (14) | 20 | 28 | 36 | 43 | 50 | |||
| ♂ 46 tergites | 13 | 20 | 27 | 33 | 41 | ||||
| ♂ 47 tergites | 13 | 19 | 27 | 35 | |||||
| ♀ 44 tergites | 13 | 21 | 28 | 35 | |||||
| j♂ 45 tergites | 13 | 19 | 25 | 33 | 39 | ||||
| ♀ 44 tergites | 13 | 18 | 24 | 30 | |||||
| ♀ 40 tergites | 13 | 19 | 25 | 34 | |||||
| B: | |||||||||
| ♀ 65 tergites | 8 | 6 | 9 | 7 | 3 | 7 | 4 | 3 | |
| ♂ 63 tergites | 7 | 8 | 8 | 7 | 6 | 4 | |||
| ♀ 63 tergites | 6 | 7 | 8 | 8 | 7 | 6 | 4 | 3 | |
| ♂ 56 tergites | 6 | 8 | 8 | 7 | 7 | ||||
| ♂ 46 tergites | 7 | 7 | 6 | 8 | |||||
| ♂ 47 tergites | 6 | 8 | 8 | ||||||
| ♀ 44 tergites | 8 | 7 | 8 | ||||||
| j♂ 45 tergites | 6 | 6 | 8 | 6 | |||||
| ♀ 44 tergites | 5 | 6 | 6 | ||||||
| ♀ 40 tergites | 6 | 6 | 9 |
Since the colour patterns of Centrobolus vastus var. sexfasciatus and Sagmatostreptus strongylopygus could be interpreted as reflecting anamorphosis, it is natural to consider whether this might also be the case for the platydesmids and andrognathids. The little that is known about anamorphosis in Platydesmida is almost exclusively due to
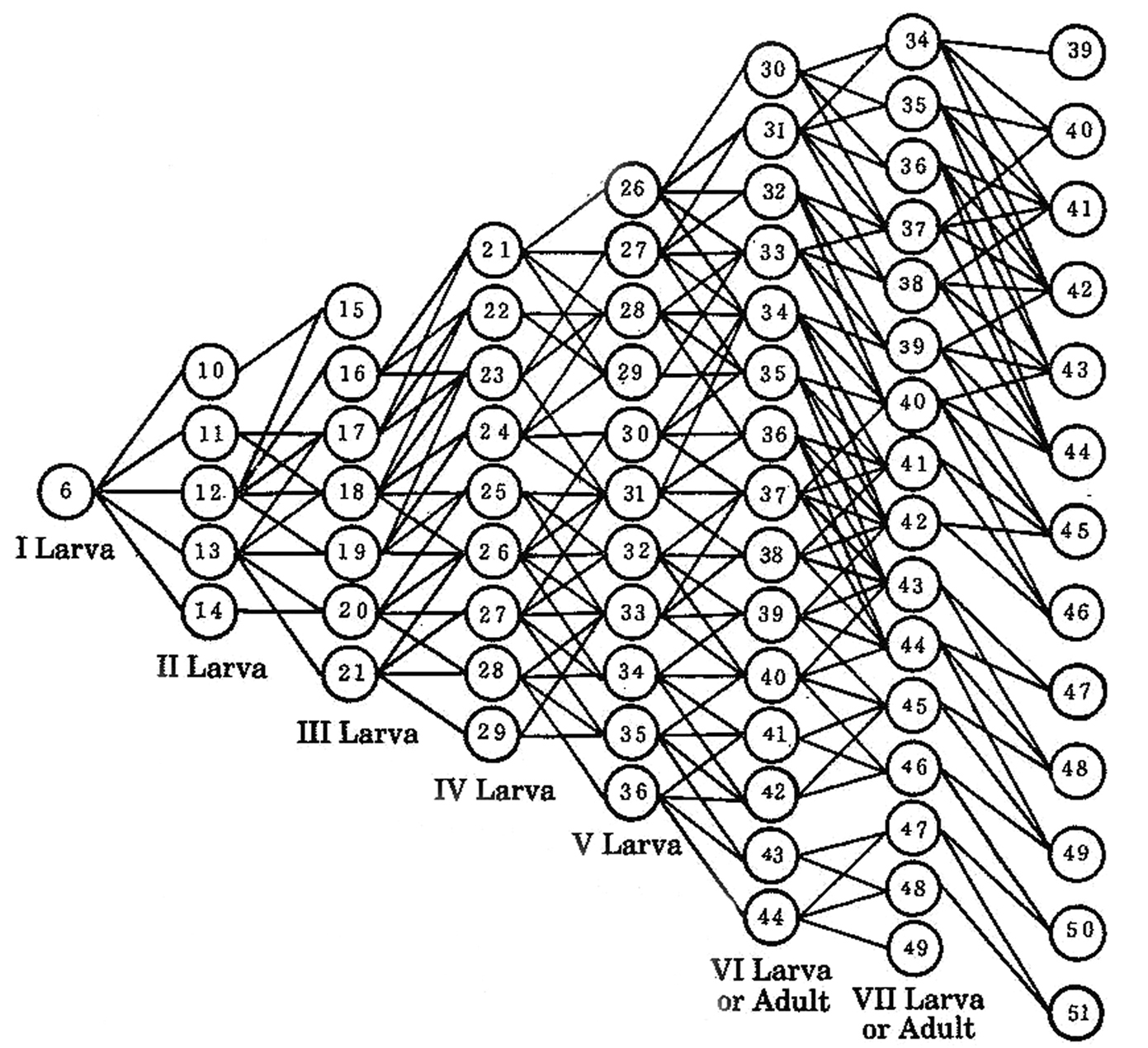
Anamorphosis in Brachycybe (=Bazillozonium) nodulosa (Verhoeff, 1935). The diagram shows the numbers of pleurotergites in the anamorphic stadia, as well as observed pathways between stadia. From
The anamorphosis of Brachycybe nodulosa is characterized by great variability:
4–8 pleurotergites are added between stadia I and II
3–8 pleurotergites are added between stadia II and III
4–8 pleurotergites are added between stadia II and IV
4–8 pleurotergites are added between stadia IV and V
3–8 pleurotergites are added between stadia V and VI
3–8 pleurotergites are added between stadia VI and VII
3–8 pleurotergites are added between stadia VII and VIII
The wide range of pleurotergal increments between stadia is consistent with the wide range of the numbers of pleurotergites which constitute a colour pattern element in the patterned platydesmids and andrognathids studied here. In other words, the colour pattern of an individual may well reflect the anamorphic pathway it has followed.
Siphonocryptus zigzagThe holotype and only known specimen of Siphonocryptus zigzag Enghoff, 2010 (order Siphonocryptida) has 32 pleurotergites and a serial colour pattern consisting of three elements covering pleurotergites 14–20, 21–27 and 28–32, respectively. The distances between colour element borders correspond to seven pleurotergites which lies within the rather wide range of distances seen in Platydesmida. However, considering the low number of pleurotergites in Siphoncryptus adults, it appears unlikely that consecutive anamorphic stadia would differ by as much as seven pleurotergites. Nothing is known about anamorphosis of the genus Siphonocryptus (the fragmentary information given by
In several species of the Australian metopidiotrichid genus Australeuma (order Chordeumatida), there is a pronounced trans-segmental pattern. For example
Fig. 10 shows two unidentified specimens of Chordeumatida from New Zealand (A. Solodovnikov and L. Vilhelmsen leg, ZMUC). They probably belong to the genus Schedotrigona (fam. Metopidiothricidae) which is the only known genus of Chordeumatida in New Zealand (
Two species of unidentified Chordeumatida from New Zealand (A. Solodovnikov & L. Vilhelmsen leg., ZMUC). Top: subadult male from South Island, Fiordland/Otago District, Lake Gunn, 44°51.500 S, 168°06.086 E, 500 masl, mixed conifer/broadleaf/beech forest, sifted leaf litter, 25.i.2011. Bottom: subadult female from South Island, Fiordland District, W slopes of Mt. Burns (W of Monowai), 45°44.808 S, 167°23.109 E, 900–950 masl, beech forest, in leaf litter and under logs, 23.i.2011. N. Akkari phot. Scales 1 mm.
No species of Metopidiotrichidae has been studied with regard to postembryonic development, but in related families, the maximal number of apodous pleurotergites is 4 (
The colour patterns described above remain exceptions among millipedes. Whereas an aposematic functon of the pattern appears obvious in Centrobolus vastus var. sexfasciatus and Sagmatostreptus strongylopygus, this is probably not the case for the patterned species of Platydesmida, Siphonocryptida and Chordeumatida. In fact, they may rather have a camouflaging function – in particular the asymmetrical pattern seen in Pseudodesmus cf. variegatus might help to dissolve the contour of the animal (I. Tuf, personal communication). The idea that the patterns reflect postembryonic development – anamorphosis – appears well-founded for Centrobolus vastus var. sexfasciatus and Sagmatostreptus strongylopygus and reasonable for the species of Platydesmida and ?Schedotrigona. For Siphonocryptus zigzag and Australeuma spp. this explanation probably does not apply.
Specimens were made available by Didier VandenSpiegel, Alexey Solodovnikov, Lars Vilhelmsen and Jan Pedersen. Guido Coza, Paul Marek and Piyatida Pimvichai gave access to photos of live animals. Nesrine Akkari and Nicolas Ioannou provided photos of preserved specimens. Richard Hoffman kindly agreed to help to make the genus name Sagmatostreptus available. Peter Decker provided insight into the SE Asian andrognathids. Ivan Tuf suggested a possible cryptic function of the asymmetrical pattern in Pseudodesmus cf. variegatus. I extend my thanks to all these persons.