(C) 2012 Monica A. Farfan. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Introduced millipede species in the family Julidae are common in the U.S. but little is known about how they interact with other organisms, such as mites. To start to determine the nature of the relationship, millipedes were sampled from across the eastern U.S.A. and the United Kingdom in 2008–2009. Sixteen morphospecies of mites (Acari: Astigmata, Mesostigmata) were collected from these millipedes, 12 of which from a total of 13 species of julid millipedes. None of these 12 species was restricted to a single host species. However, 12 of the 16 mite species collected were restricted to either the U.S.A. or the U.K. These results are consistent with locality, rather than host, specificity.
Phoresy, host specificity, Astigmata, Mesostigmata
Mites evolved relationships with other arthropods between 100–300 million years ago with the diversification of both plants and arthropods in the late Mesozoic era (
Literature records of mesostigmatid mites (Acari: Mesostigmata) associated with Diplopoda. Host names are given as in the original references.
| Mite taxon | Millipede host | Country | Source |
|---|---|---|---|
| Gamasina | |||
| Ascidae | |||
| Asca aphidioides | Parafontaria sp. | Japan |
|
| Blattisociidae | |||
| Lasioseius angustus | “Phyodesmus” sublimbatus | Indonesia |
|
| Lasioseius frontalis | Platyrrhachus mirandus | Indonesia |
|
| Lasioseius polydesmophilus | Platyrrhachus mirandus | Indonesia |
|
| Lasioseius sugawari | Oxidus gracilis | Japan |
|
| Iphiopsididae | |||
| Iphiopsis mirabilis | millipede | Italy | Berlese 1882 |
| Iphiolaelaps myriapoda | millipedes | Queensland (Australia) |
|
| Jacobsonia africanus | Spirostrepta sp. | Cameroon |
|
| Jacobsonia andrei | Spirostrepta sp. | Cameroon |
|
| Jacobsonia audyi | Thyropygus sp. | Malaya |
|
| Jacobsonia berlesei | Indo-Malayan millipede | Java-Malaysia |
|
| Jacobsonia puylaerti | Pachybolus macrosternus | Dem. Rep. Congo |
|
| Julolaelaps buensis | millipede | Cameroon |
|
| Julolaelaps cameroonensis | millipede | Cameroon |
|
| Julolaelaps celestiae | Archispirostreptus gigas | East Africa |
|
| Julolaelaps dispar | juliform millipede | Somalia |
|
| Julolaelaps excavatus | large “julid” | Dem. Rep. Congo |
|
| Julolaelaps idjwiensis | large “julid” | Dem. Rep. Congo |
|
| Julolaelaps kilifiensis | spirostreptid millipede; Archispirostreptus gigas | Kenya; unknown |
|
| Julolaelaps lucator | juliform millipede | Somalia |
|
| julid millipede | India |
|
|
| Julolaelaps madiakokoensis | large “julid” | Dem. Rep. Congo |
|
| Julolaelaps moseri | spirostreptid millipede; Archispirostreptus gigas | Trinidad; unknown |
|
| Julolaelaps myriapodalis | spirostreptid millipede | West Africa |
|
| Julolaelaps nishikawai | Nedyopus patrioticus | Japan |
|
| Julolaelaps pararotundatus | spirostreptid millipede | West Africa |
|
| spirostreptid millipede | Kenya |
|
|
| Julolaelaps parvitergalis | Parafontaria sp. | Japan |
|
| Julolaelaps parvunglatus | Parafontaria sp. | Japan |
|
| Julolaelaps paucipilis | large juliform millipede | Dem. Rep. Congo |
|
| Julolaelaps peritremalis | spirostreptid millipede | West Africa |
|
| Julolaelaps rotundatus | juliform millipede | Somalia |
|
| Julolaelaps serratus | millipede | Cameroon |
|
| Julolaelaps spirostrepti | “spirostreptus” sp. | Tanzania |
|
| Julolaelaps tritosternalis | Ommatojulus caspius | Iran |
|
| Julolaelaps vandaelensis | millipede | Cameroon |
|
| Narceolaelaps americanus | Narceus americanus | North Carolina (USA) |
|
| Narceolaelaps annularis | Narceus annularis | eastern USA |
|
| Narceolaelaps burdicki | Tylobolus sp. | California (USA) |
|
| Narceolaelaps gordanus | Narceus gordanus | Florida (USA) |
|
| Scissuralaelaps bipartitus | millipede on orchid | Philippines |
|
| Scissuralaelaps breviseta | Trigoniulus sp. | Philippines |
|
| Scissuralaelaps grootaeri | unidentified “Iule” | New Guinea |
|
| Scissuralaelaps hirschmanni | Polyconoceras sp. | New Guinea |
|
| Scissuralaelaps irianensis | unidentified millipede | New Guinea |
|
| Scissuralaelaps joliveti | Polyconoceras sp. | New Guinea |
|
| Trichaspis julus | Julus terrestris | China |
|
| Laelapidae | |||
| Cosmolaelaps hortensis | Oxidus gracilis | Japan |
|
| Hypoaspis polydesmoides | polydesmid millipede | Malaya |
|
| Iphidolaelaps myriapoda | millipede | Australia |
|
| Macrochelidae | |||
| Macrocheles muscaedomesticae | Parafontaria sp. | Japan |
|
| Ologamasidae | |||
| Stylochyrus rarior | polydesmid millipede | Iowa & Missouri (USA) |
|
| Xystodesmidae: Apheloria, Appalachioria, Brachoria, Dixioria, Nannaria, Pleuroloma, Prionogonus, Sigmoria | Appalachian Mtns. (USA) |
|
|
| Parholaspidae | |||
| Holaspulus tenuipes | Parafontaria sp. | Japan |
|
| Sejina | |||
| Heterozerconidae | |||
| Afroheterozercon pachybolus | Pachybolus macrosternus | Dem. Rep. Congo |
|
| Afroheterozercon spirostreptus | Spirostreptus cornutus | Dem. Rep. Congo |
|
| Asioheterozercon audax | Spirostreptus | Java (Indonesia) | Berlese 1910 |
| millipede | Malaysia |
|
|
| Allozercon sp. | Rhinocricidae | Philippines |
|
| “Heterozercon” elapsus | Thyropygus sp. | Sumatra (Indonesia) |
|
| Heterozercon microsuctus | spirostreptid | Brazil |
|
| Maracazercon joliveti | spirostreptid | Brazil |
|
| Narceoheterozercon ohioensis | Narceus annularis | Ohio (USA) |
|
| Trigynaspida | |||
| Costacaridae | |||
| Costacarus reyesi | millipede | Mexico |
|
| Euzerconidae | |||
| Neoeuzercon diplopodophilus | millipede | Panama |
|
| Diplogyniidae | |||
| Cryptometasternnum queenslandense | pill millipedes | Australia |
|
| Diplogynium acuminatum | millipede | Brazil |
|
| Neodiplogynium schubarti | Sooretama aguirrei | Brazil |
|
| Neotenogyniidae | |||
| Neotenogynium malkini | Orthoporus sp. | Ecuador |
|
| Paramegistidae | |||
| Meristomegistus vazquezi | ceratophallus sp. | Mexico |
|
| Neomegistus julidicola | juliform millipede | South Africa |
|
| Neomegistus remus | Proporobolus sp. | Australia |
|
| Paramegistus confrater | juliform millipede | South Africa |
|
Most mites associated with small millipedes (length < 3 cm) are not Mesostigmata, but belong to the cohort Astigmata. Reported associations of astigmatid mite and millipedes are shown in Table 2. The relationships between astigmatid mites and small millipedes are suspected to be commensalistic (
Literature reports of astigmatid mites (Acari: Astigmata) associated with Diplopoda. Host names are given as in the original references.
| Mite taxon | Millipede host | Country | Source |
|---|---|---|---|
| Histiostomatidae | |||
| Histiostoma feroniarum | Ommatoiulus moreleti | Australia |
|
| Acaridae | |||
| Caloglyphus julidicolus | Doratogonus flavifilis | South Africa |
|
| Schwiebea sp. | Xystodesmidae: Apheloria, Appalachioria, Brachoria, Boraria, Dixioria, Nannaria, Rudiloria, Sigmoria | Appalachian Mtns. (U.S.A.) |
|
| Schwiebea nova | Cylindroiulus sp. | Hungary |
|
| Canestriniidae | |||
| Diplopodocoptes transkeiensis | glomerid millipede | South Africa |
|
| Odontopygidae | Kenya |
|
|
| Chetochelacaridae | |||
| Chetochelacarus mamillatus | julid millipede | Dem. Rep. Congo |
|
| Lophonotacaridae | |||
| Lophonotacarus minutus | glomerid millipede | South Africa |
|
| Odontopygidae | Kenya |
|
|
| “Astigmata” | Polydesmus inconstans | Michigan (U.S.A.) |
|
The focal taxon for this study are millipedes in the family Julidae. These millipedes, are relatively small (20–40mm), are primarily distributed in the Palaearctic region, and are now found in a variety of anthropogenic habitats in temperate regions. They have also been recorded from many countries in Europe and from Canada, the Tristan da Cunha Island group in the Atlantic Ocean, Mexico, Chile, Peru, New Zealand, South Africa, and Antarctica (
Between March 2008 and October 2009, millipedes of the family Julidae were collected for a population genetics study. This study allows us to present preliminary data on the following questions:
1. What species are present across the eastern U.S.?
2. What mites are associated with these species?
3. Are there strong indications of host and /or locality specificity?
Host specificity in relationships between the millipedes in the family Julidae and the phoretic mite would be indicated if particular mite species are consistently associated with specific millipede host species. If mites are host specific and there was a founder effect during colonization of the New World, diversity of specific mites in the U.S. is likely to be less than in Europe. Alternately, the presence of mites on a millipede may be based on locality, not host, specificity. This assumes that mites are specific to a certain area or type of off-host habitat. In this case a wide range of hosts occurring in the preferred habitat/locality might be suitable as phoretic host. Predictions for the locality hypothesis are (1) individual mite species will be present on a number of different millipede host species but restricted to certain collection localities; (2) ‘American’, not European, mites will be present on U.S. representatives of European millipede species, and (3) there will be similar mite diversity on U.S. and European populations of the same millipede species.
Material and methods Collection localitiesMillipede specimens were collected from localities in the eastern United States from March through October in 2008 and 2009 and in the United Kingdom in April 2009. Sites in the U.S.A. were chosen to include geographic diversity and to include a number of cities used as colonial ports. While known to be found near such port cities, the current range of julid millipedes is more extensive.
Collection methodsMillipedes were collected by hand except in one instance (Whetstone Park, Columbus, OH) where litter was returned to the Ohio State University Acarology Laboratory (OSAL) for extraction by Berlese funnel. Hand collection was done from wood mulch, leaf litter, and soil. Much collecting was done at the soil/litter interface. Millipedes (with mites associates attached) were collected and placed individually into 1.5 mL vials containing 95% ethanol. Mites release their hold on immersion in alcohol, so individual host preservation is essential. Millipede and mite specimens used in this study are deposited at OSAL. Voucher numbers for representatives of all millipede host species and mite morphospecies, as well as species citations, are listed in Appendices 1 and 2.
DeterminationsMillipedes were dissected and, in some cases, genitalia were slide mounted. Specimens were identified using the following keys:
Mites were sorted to morphospecies using a 12–110× Nikon SMZ dissecting microscope, and some individuals from each morphospecies (including a diversity of hosts and/or sites) were slide-mounted for final identification using a Zeiss Axioskop compound microscope at magnifications up to 1000×. Overall, 148 mites, approximately 11% of total mites collected, were slide mounted. Astigmatid mites were identified to genus using keys by OConnor (unpublished). Specific identification proved impossible as most mite species collected appear to be undescribed. Full description of these mites is beyond the scope of this study, but we do provide some of the characters on which discrimination into morphospecies was based. This should allow evaluation of the validity of those morphospecies concepts.
Prevalence and intensityPrevalence of the identified mites was calculated for each host species and each locality. Prevalence is defined as: (number of hosts with a particular parasite species) / (number of hosts examined) (
Twenty three species of millipedes were collected representing 9 different families (Table 3). Thirteen of these species belong to the family Julidae, the focus of this study. Six were collected in large numbers (> 100); Brachyiulus pusillus, Cylindroiulus caeruleocinctus, Cylindroiulus latestriatus, Cylindroiulus punctatus, Cylindroiulus truncorum, and Ophyiulus pilosus. Millipedes in the remaining 8 families (Abacionidae, Blaniulidae, Cleidogonidae, Euryuridae, Glomeridae, Parajulidae, Polydesmidae, and Spirobolellidae), with the exception of Blaniulidae, were collected in relatively low numbers.
Intensity (over all mite morphospecies) and prevalence (percentage by mite morphospecies) for mites associated with millipedes examined.
| Family | Species | N (millipedes) | Average intensity | Rhizoglyphus A | Rhizoglyphus B | Sancassania A | Sancassania B | Schwiebea A | Schwiebea C | Schwiebea D | Schwiebea E | Schwiebea F | Schwiebea G | Schwiebea H | Thyreophagus sp. | Histiostoma sp. | Cosmolaelaps sp. | Holostapis sp. | Phaulodinychus sp. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abacionidae | Abacion lactarium | 8 | 3.0 | 13.0 | 13.0 | ||||||||||||||
| Blaniulidae | Blaniulid sp. | 104 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | ||||||||||||
| Blaniulus guttulatus | 93 | 1.2 | 4.0 | 1.0 | |||||||||||||||
| Choneiulus palmatus | 8 | 2.4 | |||||||||||||||||
| Nopoiulus kochii | 76 | 1.6 | |||||||||||||||||
| Cleidogonidae | Cleidogona caroliniana | 7 | 2.0 | ||||||||||||||||
| Euryuridae | Euryurus leachii | 14 | 11.3 | 7.0 | 7.0 | 7.0 | 14.0 | 14.0 | |||||||||||
| Glomeridae | Glomeris marginata | 4 | 2.7 | 5.0 | |||||||||||||||
| Julidae | Brachyiulus lusitanus | 16 | 1.0 | ||||||||||||||||
| Brachyiulus pusillus | 208 | 2.3 | 1.0 | 2.0 | |||||||||||||||
| Cylindroiulus britannicus | 35 | 2.0 | 3.0 | 3.0 | 3.0 | 3.0 | |||||||||||||
| Cylindroiulus caeruleocinctus | 365 | 2.8 | 2.0 | 1.0 | 0.3 | 0.3 | 1.0 | ||||||||||||
| Cylindroiulus latestriatus | 337 | 1.7 | 0.3 | 0.3 | 0.3 | 0.3 | |||||||||||||
| Cylindroiulus londinensis | 2 | 5.0 | 50.0 | 50 | |||||||||||||||
| Cylindroiulus punctatus | 109 | 3.3 | 1.0 | 4.0 | 1.0 | 1.0 | |||||||||||||
| Cylindroiulus truncorum | 171 | 1.9 | 1.0 | 2.0 | 1.0 | 1.0 | |||||||||||||
| Cylindroiulus sp. | 186 | 2.7 | 1.0 | 2.0 | 1.0 | ||||||||||||||
| Julus scandinavicus | 19 | 3.2 | 5.0 | 5.0 | |||||||||||||||
| Ommatoiulus sabulosus | 9 | 2.0 | 11.0 | ||||||||||||||||
| Ophyiulus pilosus | 373 | 2.6 | 0.3 | 1.0 | |||||||||||||||
| Tachypodoiulus niger | 2 | 5.0 | 50.0 | ||||||||||||||||
| Parajulidae | Uroblaniulus carolinensis | 8 | 2.7 | 13.0 | 13.0 | ||||||||||||||
| Polydesmidae | Polydesmus angustus | 6 | 6.0 | 17.0 | 17.0 | 17.0 | 17.0 | ||||||||||||
| Polydesmus sp. | 8 | 1.0 | 13.0 | ||||||||||||||||
| Spirobolellidae | Paraspirobolus lucifugus | 5 | 2.9 | 20.0 | 40.0 |
Representatives of 6 genera of mites were associated with millipedes collected in this study. These mites belong to two families in the cohort Astigmata; Acaridae and Histiostomatidae, and two families in the suborder Mesostigmata; Laelapidae and Uropodidae.
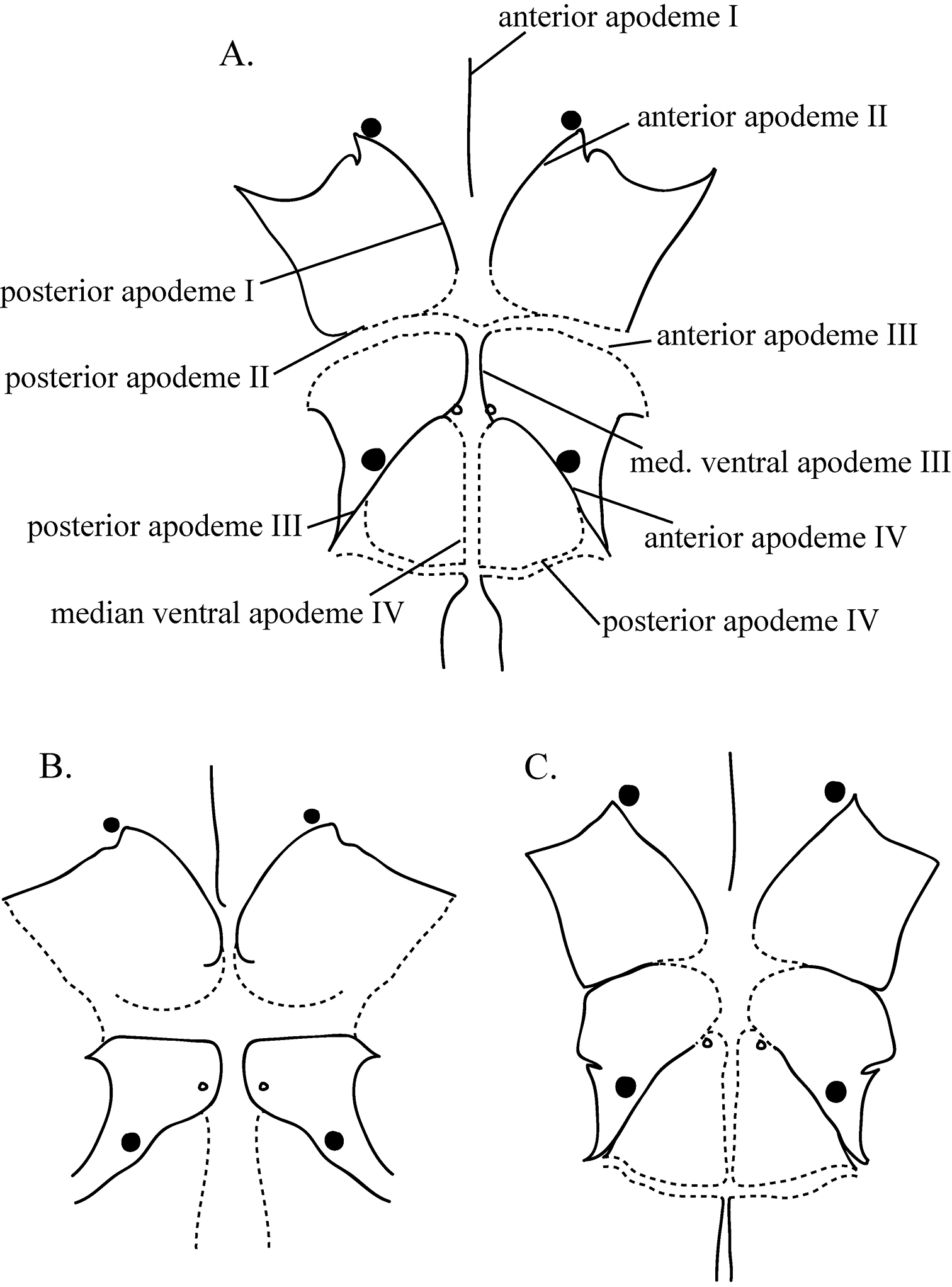
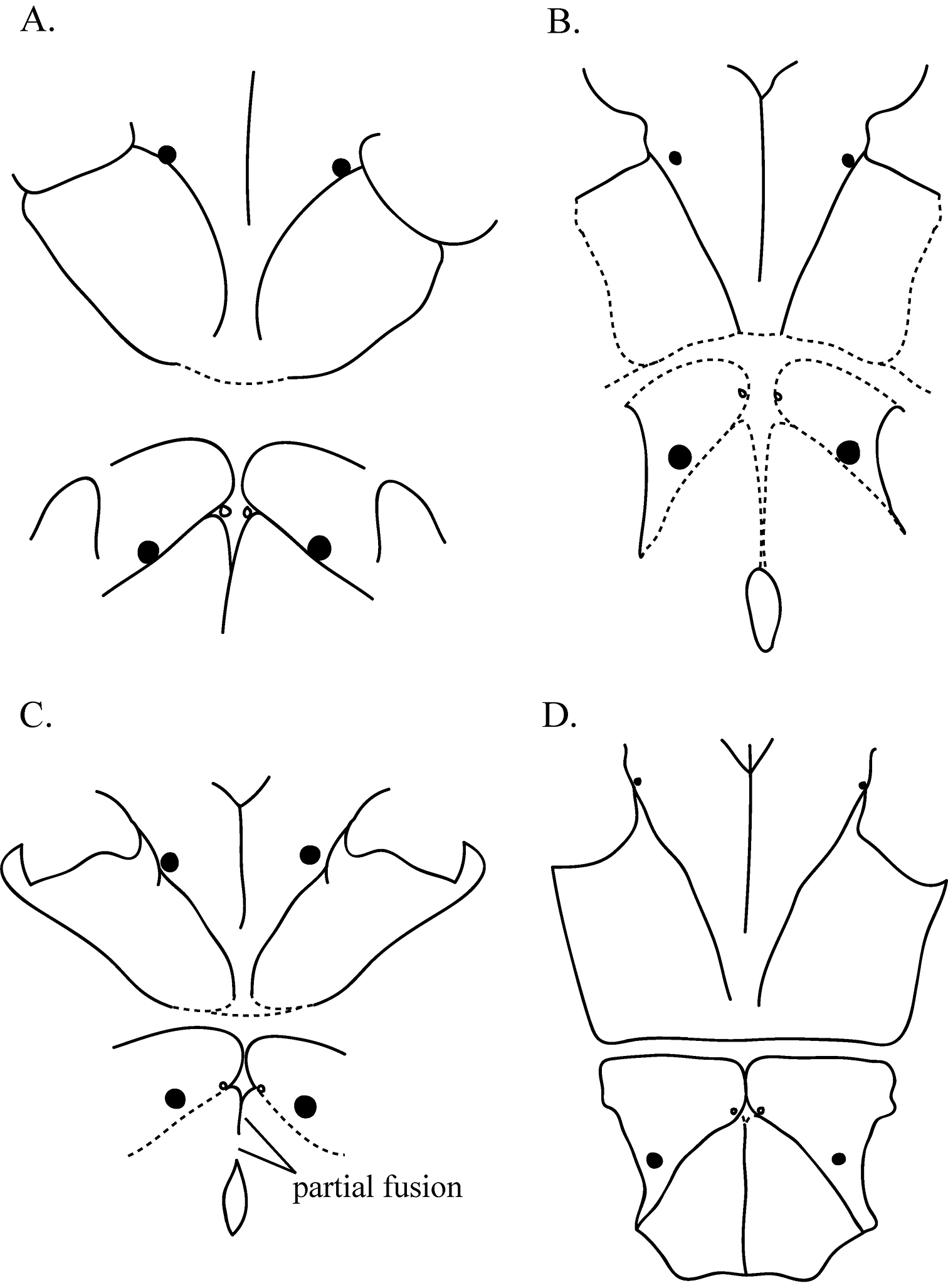
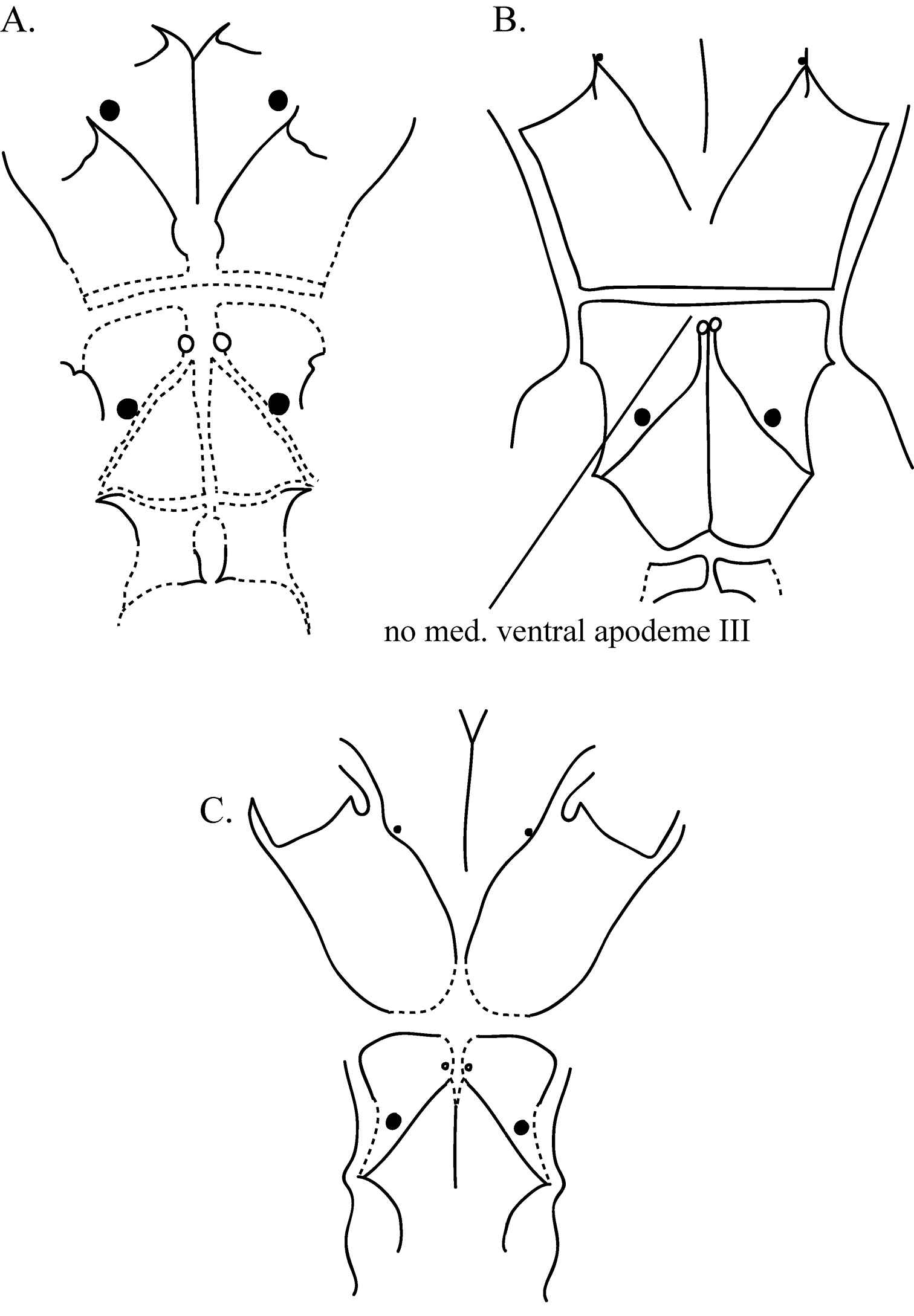
AstigmataThe astigmatid mites represented 13 morphospecies: Rhizoglyphus A and B (Figure 1a and 1c, respectively), Sancassania A and B (Figure 1b), Schwiebea A, C, D, E (Figures 2a, 2b, 2c, & 2d, respectively), F, G, and H (Figures 3a, 3b, & 3c, respectively), Thyreophagus (all Acaridae), and Histiostoma (Histiostomatidae). Specimens of Rhizoglyphus, Sancassania, and Schwiebea were separated into morphospecies by the shape and amount of fusion of the apodemes and the shape of the coxal fields on the venter. These characteristics were chosen because these features are unlikely to be artifacts of the slide-mounting process. The differences between the species can be subtle. Distinguishing characteristics of the morphospecies within the genera Rhizoglyphus, Sancassania, and Schwiebea are detailed in Table 4, and Figures 1–3. Figures show conoidal setae as filled-in circles and simple setae as open circles. The size of the filled-in circles in the diagram is indicative of the relative size of the conoidal setae. Broken lines indicate edges of apodemes with a greater degree of fusion. These edges appear not as distinct or “heavy” as non-fused apodemes. Apodemes and coxal fields are denoted with Roman numerals corresponding with the leg pair they are associates with (e.g., coxal fields II correspond with legs II).
Diagram of the coxal fields and coxal apodemes of a Rhizoglyphus A b Rhizoglyphus B and c Sancassania B.
Diagram of the coxal fields and coxal apodemes of a Schwiebea A b Schwiebea C c Schwiebea D and d Schwiebea E.
Diagram of the coxal fields and coxal apodemes of a Schwiebea F b Schwiebea G and c Schwiebea H.
Comparative characters for Schwiebea (Acari, Astigmata, Acaridae) species associated with millipedes (Diplopoda).
| Schwiebea A (Fig. 2A) | Schwiebea C (Fig. 2B) | Schwiebea D (Fig. 2C) | Schwiebea E (Fig. 2D) | Schwiebea F (Fig. 3A) | Schwiebea G (Fig. 3B) | Schwiebea H (Fig. 3C) | |
|---|---|---|---|---|---|---|---|
| Coxal fields II | oval, well-developed | quadrilateral; apodemes straight, well-developed | oval, widened distally, mostly well-developed | quadrilateral; coxal fields I & II contiguous, well-developed | quadrilateral; anterior apodemes II end in distinct curve medially | quadrilateral; fields I & II contiguous, well-developed | oval, well-developed |
| Posterior edge of coxal fields II | convex, poorly developed medially | concave, poorly developed | convex, poorly developed medially | straight, horizontal, well-developed | slightly concave, quadrilateral edge poorly developed | straight, horizontal, well-developed | median section of posterior curve poorly developed |
| Anterior edge of coxal fields III | roughly concave, well-developed | concave, poorly developed | slightly concave, well-developed | straight, horizontal, well-developed | slightly concave, poorly developed | straight, horizontal, well-developed | slightly concave at lateral edges, well-developed |
| Coxal fields III | rounded medially | rounded medially | rounded medially, almost touching | triangular, separated medially | triangular, not touching | connected, quadrilateral | triangular, not touching |
| Coxal fields IV | triangular | triangular | triangular | deltoid | triangular | deltoid; acutely tapering medially | arrow-shaped |
| Apodemes III & IV | well-developed | poorly developed | anterior III and median ventral apodemes well-developed; others poorly developed | well-developed | poorly developed | well-developed | mostly well-developed |
| Median ventral apodemes III | not fused | not fused | almost fused | fused | not fused | not present | not fused, poorly developed |
| Median ventral apodemes IV | fused, extending to genital opening | not fused, extending to genital opening | fused, terminates half-way to genital opening | fused, extending to genital opening | not fused, extending to genital opening | fused, extending to genital opening | fused, terminates half-way to genital opening |
| Conoidal setae on coxal field I | medium | medium | large | small | large | very small | very small |
Mites in the families Laelapidae and Uropodidae were determined to genus. Two genera of laelapids were identified as Cosmolaelaps and Holostaspis, the uropodid as Phaulodinychus.
Julidae - mite associationsNearly all mite specimens collected from julid millipedes were astigmatid deutonymphs. Morphospecies of Schwiebea were the most commonly collected from these millipedes (Table 3). Rhizoglyphus A was collected from the highest number of julid species in this study (n = 9). This was followed by Histiostoma sp. (n = 6). Thyreophagus specimens were found on Ommatoiulus sabulosus and Cylindroiulus britannicus only. Schwiebea D, E, and G and Phaulodinychus sp. were also collected from one julid host species only. Phaulodinychus sp. was collected only once on a julid millipede, Cylindroiulus latestriatus. There were no laelapid mites collected from julid millipedes.
Cylindroiulus caeruleocinctus had five species of mite associates which was the highest diversity among julids. Tachypodoiulus niger and Ommatoiulus sabulosus had the fewest associate species (one species each).
Prevalences were found to range from 0.27%–50.0% (minimum estimates). Notable are the associations of Schwiebea H on Tachypodoiulus niger (N=2), and Rhizoglyphus A and Histiostoma sp. on Cylindroiulus londinensis (N= 2), each with the highest prevalence of 50.0%. The lowest prevalence was calculated for Rhizoglyphus Aon Ommatoiulus pilosus (0.27%) (but see comments in Material and Methods). Intensity also tends to be low. The average intensities for the six most common julid species ranged from 1.66–3.32 mites per host with an average of 2.43. This is probably close to the average intensity for all Julidae.
Non-julid millipede collectionsSchwiebea C was collected from the highest number of non-julid hosts (n = 5). Polydesmus angustus was associated with 4 morphospecies of Schwiebea, all collected from one host individual. Histiostoma sp. and three species of Schwiebea have been collected from the combined specimens in the family Blaniulidae. The two taxa collected from Uroblaniulus carolinenesis and Paraspirobolus lucifugus were Histiostoma sp. and Schwiebea C. Abacion lactarium was the only species, julid or non-julid, to have Rhizoglyphus B as an associate. The euryurid Euryurus leachii was the only millipede host to have mite associates in the family Laelapidae. Representatives of two genera, Cosmolaelaps and Holostaspis, were collected. Consistent with the general association pattern of Laelapidae, these mites were collected as adults, not deutonymphs. Additionally, a considerable number of deutonymphs of the uropodine Phaulodinychus sp. were collected from this millipede. Euryurus leachii was the millipede with the highest intensity (11.27) and the largest number of mite species among the non-julids with five mite morphospecies.
The prevalence of mites found on non-julid millipedes was generally high. This may be associated with the fact that the numbers of non-julids collected were relatively low, with the exception of blaniulids. For example, 50.0% of the Glomeris marginata (N = 4) carried Schwiebea E. Blaniulids were collected most commonly, and showed prevalences between 0.94–3.77% for their mite associates Schwiebea D, E, and F, and Histiostoma sp.
Mite taxa and telation to localityOnly four mite species were collected from localities in both the U.S.A. and in the U.K., Rhizoglyphus A, Schwiebea C, Schwiebea F, and Histiostoma sp. (Table 5). Mite taxa that were found exclusively in the U.S.A. are Rhizoglyphus B, Sancassania A, Sancassania B, Schwiebea D, Cosmoglyphus sp., Holostapis sp. and Phaulodinychus sp. Taxa found only in the U.K. Schwiebea A, Schwiebea E, Schwiebea G, Schwiebea H, and Thyreophagus sp. The highest diversity of mite taxa by locality was found in Cornwall, U.K. Only one mite taxon each was collected in Chicago, IL, Columbus, OH, and Lakewood, OH.
Prevalence (percentage) of mites on millipedes (all species combined) by collecting locality.
| Locality | N (millipedes) | Rhizoglyphus A | Rhizoglyphus B | Sancassania A | Sancassania B | Schwiebea A | Schwiebea C | Schwiebea D | Schwiebea E | Schwiebea F | Schwiebea G | Schwiebea H | Thyreophagus sp. | Histiostoma sp. | Cosmolaelaps sp. | Holostapis sp. | Phaulodinychus sp. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| North Kingstown, RI | 175 | 1 | 3 | 1 | 1 | 2 | |||||||||||
| Chicago, IL | 452 | 1 | |||||||||||||||
| Lakewood, OH | 61 | 2 | |||||||||||||||
| Cleveland, OH | 119 | 2 | 1 | ||||||||||||||
| Delaware, OH | 23 | 9 | 4 | 4 | |||||||||||||
| Columbus, OH | 1054 | 1 | |||||||||||||||
| Southern Ohio | 7 | 14 | 29 | 33 | |||||||||||||
| Baltimore, MD | 102 | 8 | 1 | 3 | 2 | ||||||||||||
| Charlotte, NC | 161 | 1 | 1 | 1 | 1 | ||||||||||||
| Fort Mill, SC | 15 | 7 | 7 | 7 | |||||||||||||
| Cornwall, UK | 59 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | ||||||||
| Eden Project, UK | 56 | 2 | 4 | 2 | 2 | 5 | |||||||||||
| Slough, UK | 27 | 7 | 19 | 7 | 4 | 4 | 4 | 4 |
Of the seven millipede species collected in both the U.S.A. and the U.K. only Cylindroiulus caeruleocinctus had the same mite associates in both countries, Rhizoglyphus A and Histiostoma sp. (Table 5). Mite associates of the other six species of millipedes did not appear in both countries on the same julid host species.
DiscussionThe current study does not suggest strong host specificity of mite species for julid hosts. The only species that were collected from only one host, Rhizoglyphus B, Cosmolaelaps sp. and Holostaspis sp. are rare, and (so far) exclusively associated with non-julid hosts. This result supports the view of OConnor (1998) who noted that acarids are (1) often associates of a wide variety of hosts and (2) are often cosmopolitan. There is precedent for this in terms of millipede-associated Mesostigmata. While most Heterozerconidae are associated with large millipedes, one genus, Amheterozercon (
Neither is there strong overlap of the mite fauna of specific julid millipedes collected both in the U.S.A. and the U.K., another prediction of the host specificity hypothesis. Only one millipede species (Cosmolaelaps caeruleocinctus) carried the same mite species in both the U.S.A. and the U.K. In a comparison of mite associates found on millipedes and the locality in which they were found (Table 5), 12 of the 16 taxa were collected in either the U.S.A. or the U.K. Only four were collected from both countries. This is an indication that the mites are most likely not associated with specific hosts but, instead, are favoring hosts associated with specific habitats.
An unusual finding regarding mite associates was the collection of a species in the genus Thyreophagus collected in the U. K. Past reports of Thyreophagus sp. are mostly from the U.S.A. Thyreophagus is a mite thought to be associated only with subcortical insects (
Thanks to Helen J. Read, and the British Myriapod and Isopod Group for their help in the collection of specimens, and to Barry OConnor for permission to use his unpublished keys to Astigmatid genera. Thanks to John W. Wenzel, currently Carnegie Mellon University, and Andrew P. Michel, OSU Department of Entomology, for their kind review of earlier drafts of this publication.
Voucher numbers for millipede hosts.
| Millipede species | Author, year | Accession number |
|---|---|---|
| Abacion lactarium | (Say, 1821) | N/A |
| Blaniulus guttulatus | (Fabricius, 1789) | OSAL0100704 ♂ |
| Brachyiulus lusitanus | Verhoeff, 1898 | OSAL0100959 ♂ |
| *OSAL006951 ♂ | ||
| Brachyiulus pusillus | (Leach, 1815) | OSAL0100593 ♂ |
| Choneiulus palmatus | (Nemec, 1895) | OSAL0100811 ♂ |
| Cleidogona caroliniana | Causey, 1957 | OSAL0100283 ♂ |
| Cylindroiulus britannicus | (Verhoeff, 1891) | OSAL0100455 ♀ |
| OSAL0100394 ♂ | ||
| *OSAL006949 ♀ | ||
| *OSAL006950 ♂ | ||
| Cylindroiulus caeruleocinctus | (Wood, 1864) | OSAL0100759 ♂ |
| *OSAL006948 ♂ | ||
| Cylindroiulus latestriatus | (Curtis, 1845) | OSAL0100650 ♂ |
| *OSAL006947 ♂ | ||
| Cylindroiulus londinensis | (Leach, 1815) | OSAL0100375 ♂ |
| *OSAL006955 ♂ | ||
| Cylindroiulus punctatus | (Leach, 1815) | OSAL0100417 ♂ |
| Cylindroiulus truncorum | (Silvestri, 1896) | OSAL0100935 ♀ |
| OSAL0100536 ♂ | ||
| *OSAL006952 ♂ | ||
| Euryurus leachii | (Gray, 1832) | OSAL0100840 ♂ |
| Glomeris marginata | (Villers, 1789) | OSAL0100376 ♂ |
| Julus scandinavicus | Latzel, 1884 | OSAL0100358 ♂ |
| Nopoiulus kochii | (Gervais, 1836) | OSAL0100282 ♂ |
| Ommatoiulus sabulosus | (Linné, 1815) | OSAL0100403 ♂ |
| Ophyiulus pilosus | (Newport, 1843) | OSAL0100423 ♀ |
| OSAL0100613 ♂ | ||
| *OSAL006954 ♀ | ||
| *OSAL006953 ♂ | ||
| Paraspirobolus lucifugus | (Gervais, 1836) | OSAL0100386 ♂ |
| Polydesmus angustus | (Latzel 1884) | OSAL0100439 ♂ |
| Tachypodoiulus niger | (Leach, 1815) | OSAL0100384 ♂ |
| Uroblaniulus carolinensis | Causey, 1953 | OSAL0100882 ♂ |
* denotes slide of genitalia
Voucher numbers for associated mite morphospecies.
| Mite morphospecies | Accession number |
|---|---|
| Holostapis sp. | OSAL0006958 |
| Phaulodinychus sp. | OSAL0006790 |
| Rhizoglyphus A | OSAL0083451 |
| Rhizoglyphus B | OSAL0006896 |
| Sancassania A | OSAL0006911 |
| Sancassania B | OSAL0083471 |
| Schwiebea A | OSAL0000697 |
| Schwiebea C | OSAL0006942 |
| Schwiebea D | OSAL0083456 |
| Schwiebea E | OSAL0083474 |
| Schwiebea F | OSAL0006853 |
| Schwiebea G | OSAL0083448 |
| Schwiebea H | OSAL0006939 |
| Thyreophagus sp. | OSAL0006843 |