(C) 2012 Dimitris Kaltsas. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The seasonal activity patterns of Scolopendra cingulata and Scolopendra cretica in relation to abiotic factors and microhabitat preferences in five eastern Mediterranean maquis formations were studied. The abundance of both species proved to be spatially non-variant, suggesting a uniform distribution of populations, which exhibited a statistically similar phenological pattern, peaking in early-midsummer. The variability of the temporal activity patterns in Crete, Naxos and Cyprus denotes the influence of insularity and rapid change of environmental conditions to the phenology of both species. The annually consistent seasonal activity represents an invariant pattern in continental areas such as Attiki and Samos. Although young and large adults were more abundant than juveniles, the microhabitat preferences of scolopendrids did not differ between the two species and in relation to age class and study site and did not change temporally. The correlation of abundance with high air temperature and low air relative humidity and precipitation shows that both species are thermophilous and xerophilous, well adapted to the environmental conditions of the eastern Mediterranean region.
Crete, Cyprus, Greece, Juniperus phoenicea, phenological pattern, Scolopendra
The ecosystems in the Mediterranean basin are insufficiently studied (
The centipedes of genus Scolopendra Linnaeus, 1758 are soil predators, living in moist surroundings, and are found mostly under stones and beneath the bark of decayed logs. In the field they are rarely seen above ground during daylight and are mainly active during the wet periods (spring and autumn). They live in all tropical and warm temperate areas (southern Europe, Asia, the Americas, Africa, Australia) (
Herewith we present the results of a study on the seasonal activity of Scolopendra cretica Lucas, 1853 and Scolopendra cingulata Latreille, 1829 carried out from 2006 to 2008. The former is known from Crete and its satellite islets (Simaiakis & Mylonas 2008) and its taxonomic status has been revised recently (
Maquis is a typical formation in the Mediterranean ecosystems and especially the eastern Mediterranean where it is usually mixed with phryganic species. Despite its high conservation value (
Pitfall trapping was used to study the seasonal activity of Scolopendra cingulata and Scolopendra cretica in five continental and insular ecosystems dominated by the Phoenician juniper. The role of age, microhabitats and certain climatic features (air temperature, relative humidity, and precipitation) on the seasonal activity patterns was investigated. This is the first thorough study on the phenological patterns of scolopendromorph species in the eastern Mediterranean region.
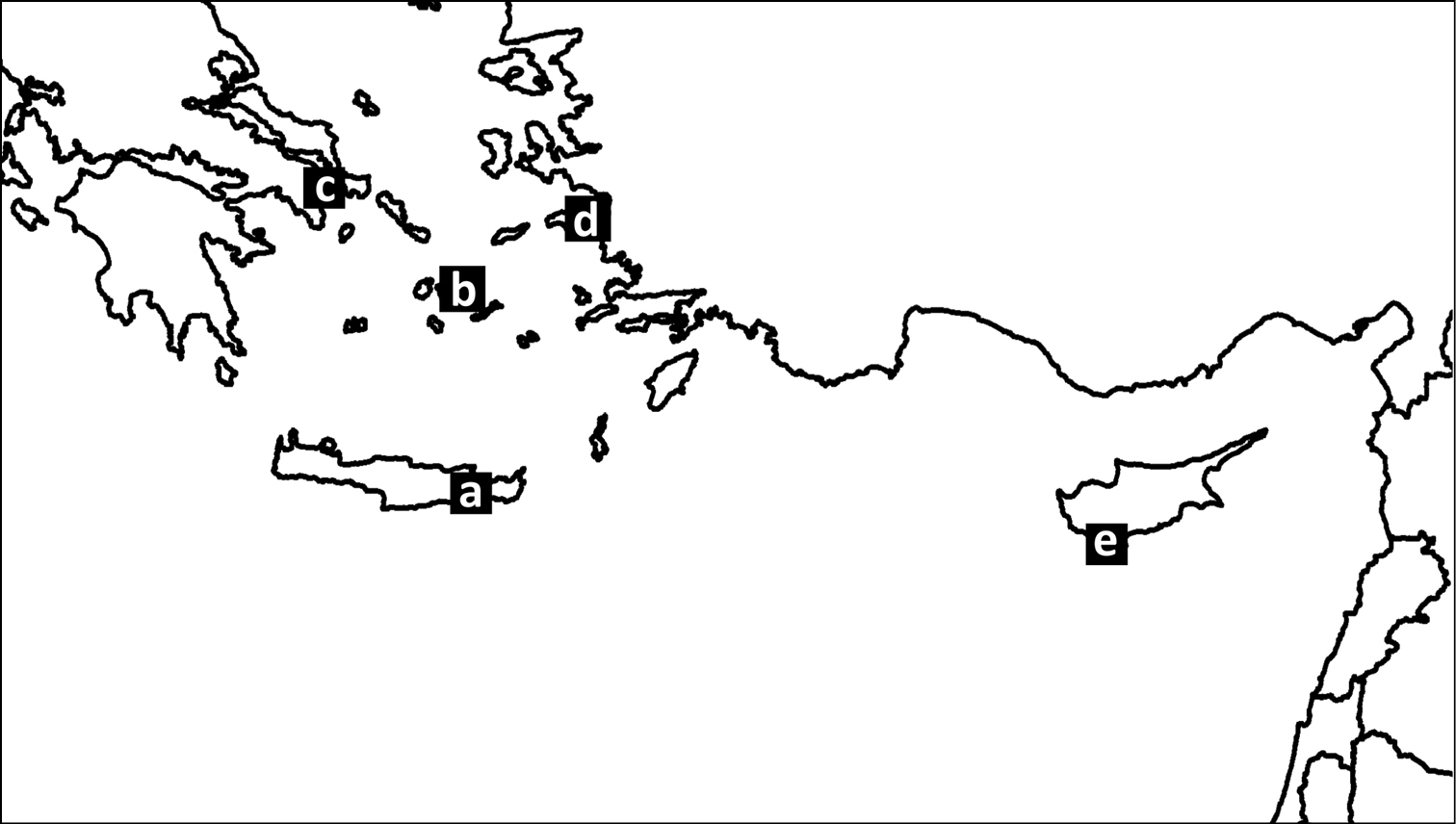
Materials and methods The study sitesThe study was conducted in five areas of the eastern Mediterranean, four of which are located in Greece and one in Cyprus. The localities of the sites are: a) Pacheia Ammos in NE Crete (35°6'35"N, 25°49'9"E), b) Moutsouna in E Naxos (37°2'46"N, 25°34'27"E), c) Agia Marina in NE Attiki (38°10'57"N, 24°3'12"E), d) Psili Ammos in SE Samos (37°42'26"N, 27°1'29"E), and e) Kourio in S Cyprus (34°40'3"N, 32°51'48"E) (Figure 1). All study sites were close to the coast (50–100 m), at an altitude of approximately 40 m. The substrate at the sites is limestone and the vegetation is characterized by the dominance of the typical maquis species Juniperus phoenicea (relative cover - RCi%: 38.4–60.9%) and Pistacia lentiscus (RCi%: 11.5–29.1%)).
The location of the five study sites in a map of the Eastern Mediterranean. a Pacheia Ammos (Crete) b Moutsouna (Naxos) c Agia Marina (Attiki) d Psili Ammos (Samos) e Kourio (Cyprus).
At all sites material was sampled using pitfall traps (depth: 11.5 cm; diameter: 9.5 cm) containing propylene glycol as a preservative. The traps at the five study sites were sampled bimonthly. The collection of material took place over two years, from the beginning of May 2006 to the beginning of May 2008. The study consisted of two phases: a) May 2006–May 2007: 20 pitfall traps were placed with 10 m trap spacing; b) May 2007–May 2008: three groups of seven traps, with 10 m trap spacing at all sites. The minimum distance between the groups was 60 m. The three groups were introduced in order to test whether the abundance of scolopendrids differed statistically within each study site. The traps covered the three major microhabitat types of the study areas; dense vegetation and litter cover (dv), scarce vegetation (sv) and open areas (o).
ClimateDuring the study air temperature and air relative humidity were measured using a MicroLog® Compact Data Logger (Fourier Systems), and precipitation using WS-7038U 433 MHz Wireless Rain Monitor® (La Crosse Technology) (see Appendix 1 for detailed climate data). Generally, the climate was typical Mediterranean with high mean monthly air temperature (20 °C) and low annual precipitation (445 mm/year), 65% of which occurred during the cold months (November-February) at all study sites.
Morphometric age traitsBecause it could be risky to determine the age for each individual, we measured two age related characters namely i) the body length and ii) the number of antennomeres of the captured scolopendrids. Both characters were measured with an ocular micrometer using a Leica MZ6 stereomicroscope. Body length from the anterior margin of the head shield to the end of the telson was measured on a millimetre paper. Regarding antennomeres, we counted the number of articles of the right antenna. Samples were split into three classes of body size: (i) juveniles (body length < 40 mm), (ii) young adults (40 < body length < 60 mm) and, (iii) large adults (body length > 60 mm). We excluded specimens of less than 16 and more than 21 antennomeres, because such measurements are considered as abnormal (
Differences in activity density of the scolopendrids (no. of individuals per 100 trap-days) at the five study sites were tested using Kruskal-Wallis ANOVA and post-hoc multiple comparisons test. To examine whether the abundance of scolopendrids differed in relation to their age class and microhabitat preference in the five sites, we used repeated measures MANOVA. We set the abundance of scolopendrids per sampling as dependent variables and age classes, microhabitat types and the study sites or the two species, as categorical predictors. The relation between the abundance of scolopendrids and classes of each abiotic factor was analyzed using Correspondence Analysis.
The phenological patterns of the two studied species were analyzed using circular statistics (
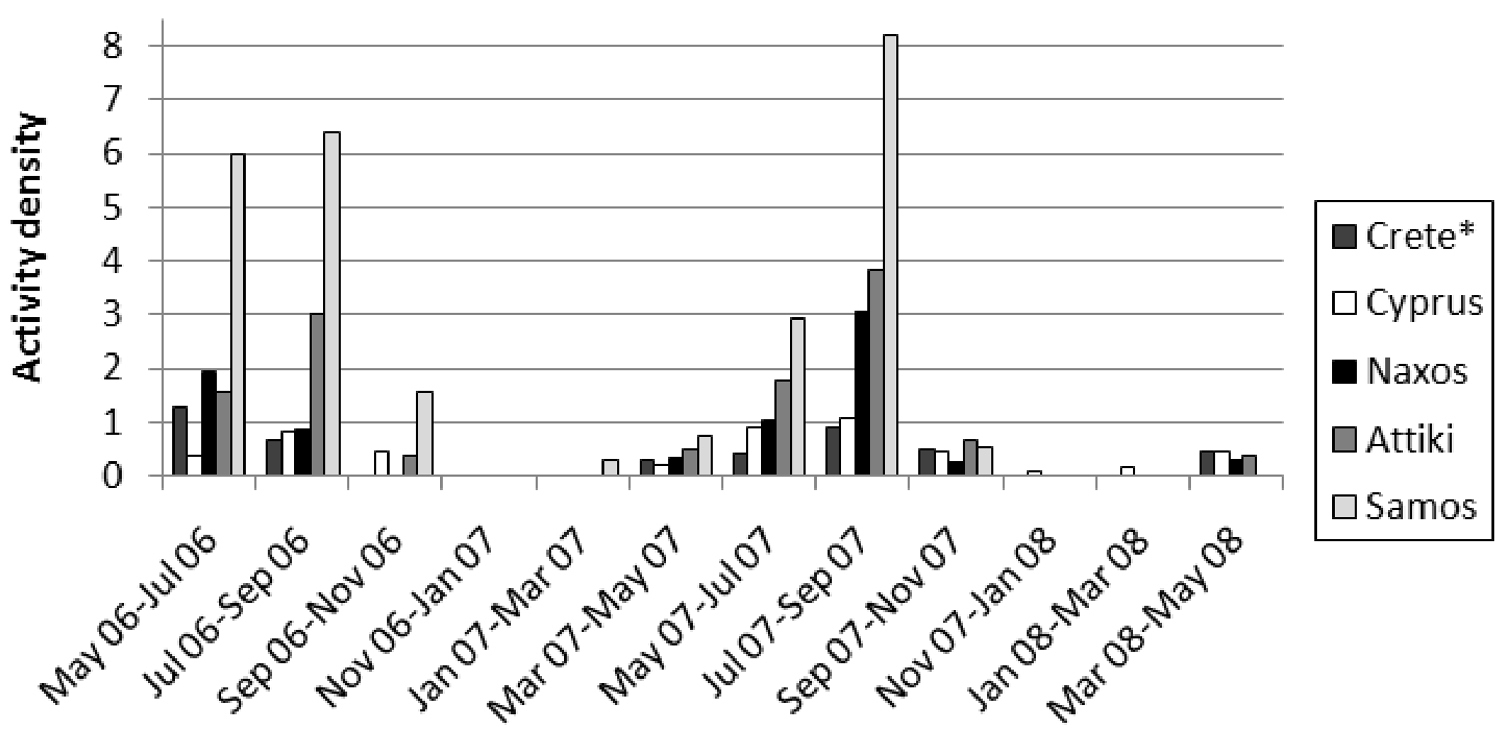
The absolute numbers of collected specimens per site and collecting periods are presented in Table 1. The abundance of active scolopendrids differed significantly between the five study sites (Kruskal-Wallis ANOVA: H = 31.047, p < 0.001), in particular between Scolopendra cretica (Crete) and Scolopendra cingulata in Samos (p < 0.001), as shown by the post-hoc multiple comparisons test. However, species-level analysis did not result in significance (H = 7.538, p = 0.06). The activity density of the two species differed neither temporally between the two years of study (8 < H < 20, 0.095 < p < 0.844) nor spatially among the three subsites within each sampling site (0 < H < 4.964, 0.084 < p < 0.95) in any of the five sampling stations. Furthermore, the abundance of scolopendrids did not differ statistically between the three microhabitat types (Table 2) in any of the study areas (0.106 < H < 1.043, 0.594 < p < 0.948); thus there was no evidence of microhabitat preference for the two species.
Absolute numbers of collected specimens per period in the five sampling sites.
| Crete | Attiki | Naxos | Cyprus | Samos | |
|---|---|---|---|---|---|
| May 06 - Jul. 06 | 9 | 19 | 24 | 5 | 76 |
| Jul. 06 - Sept. 06 | 0 | 33 | 10 | 8 | 70 |
| Sept. 06 - Nov. 06 | 0 | 5 | 0 | 6 | 23 |
| Nov. 06 - Jan. 07 | 0 | 0 | 0 | 0 | 0 |
| Jan. 07 - Mar. 07 | 0 | 0 | 0 | 0 | 3 |
| Mar. 07 - May 07 | 3 | 6 | 4 | 3 | 9 |
| May 07 - Jul. 07 | 4 | 19 | 11 | 12 | 35 |
| Jul. 07 - Sept. 07 | 8 | 22 | 40 | 8 | 84 |
| Sept. 07 - Nov. 07 | 6 | 5 | 3 | 3 | 4 |
| Nov. 07 - Jan. 08 | 0 | 0 | 0 | 1 | 0 |
| Jan. 08 - Mar. 08 | 0 | 0 | 0 | 2 | 0 |
| Mar. 08 - May 08 | 6 | 5 | 4 | 6 | 0 |
| Total specimens | 36 | 114 | 96 | 54 | 304 |
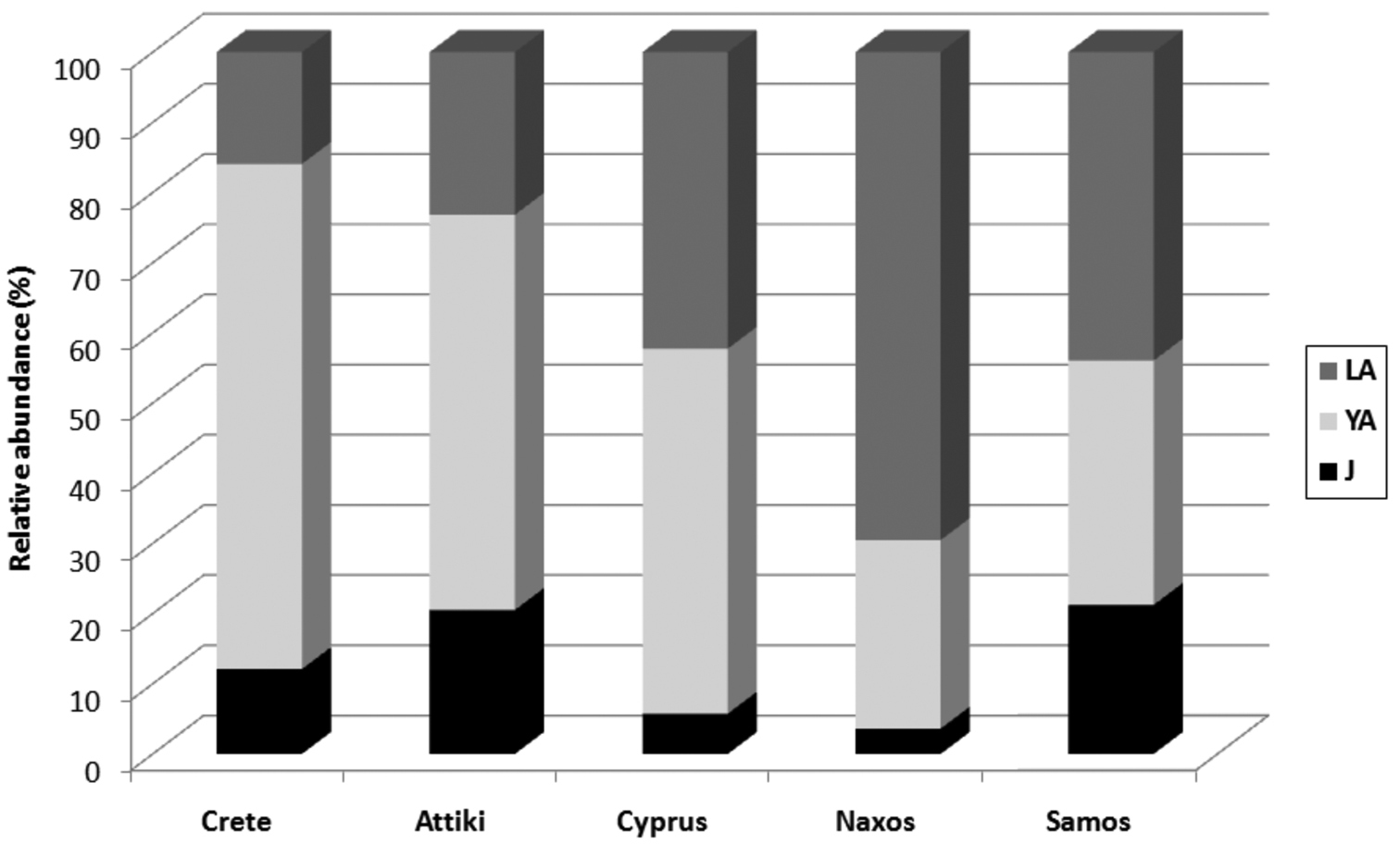
Young adult scolopendrids were the most abundant age class in Crete, Attiki and Cyprus, whereas large adults were dominant in Naxos and Samos, and juveniles were the least abundant age class at all study sites (Figure 2). The difference in catchability among the three age classes in relation to study site was proved statistically by repeated measures MANOVA (F = 1.99, p = 0.001). The same analysis showed that the microhabitat preferences of scolopendrids did not differ in relation to site, species or age class (0.803 < F < 1.044, 0.41 < p < 0.815).
Relative abundance of scolopendrid age classes in the five study sites. J; juvenile, YA; young adult, LA; large adult.
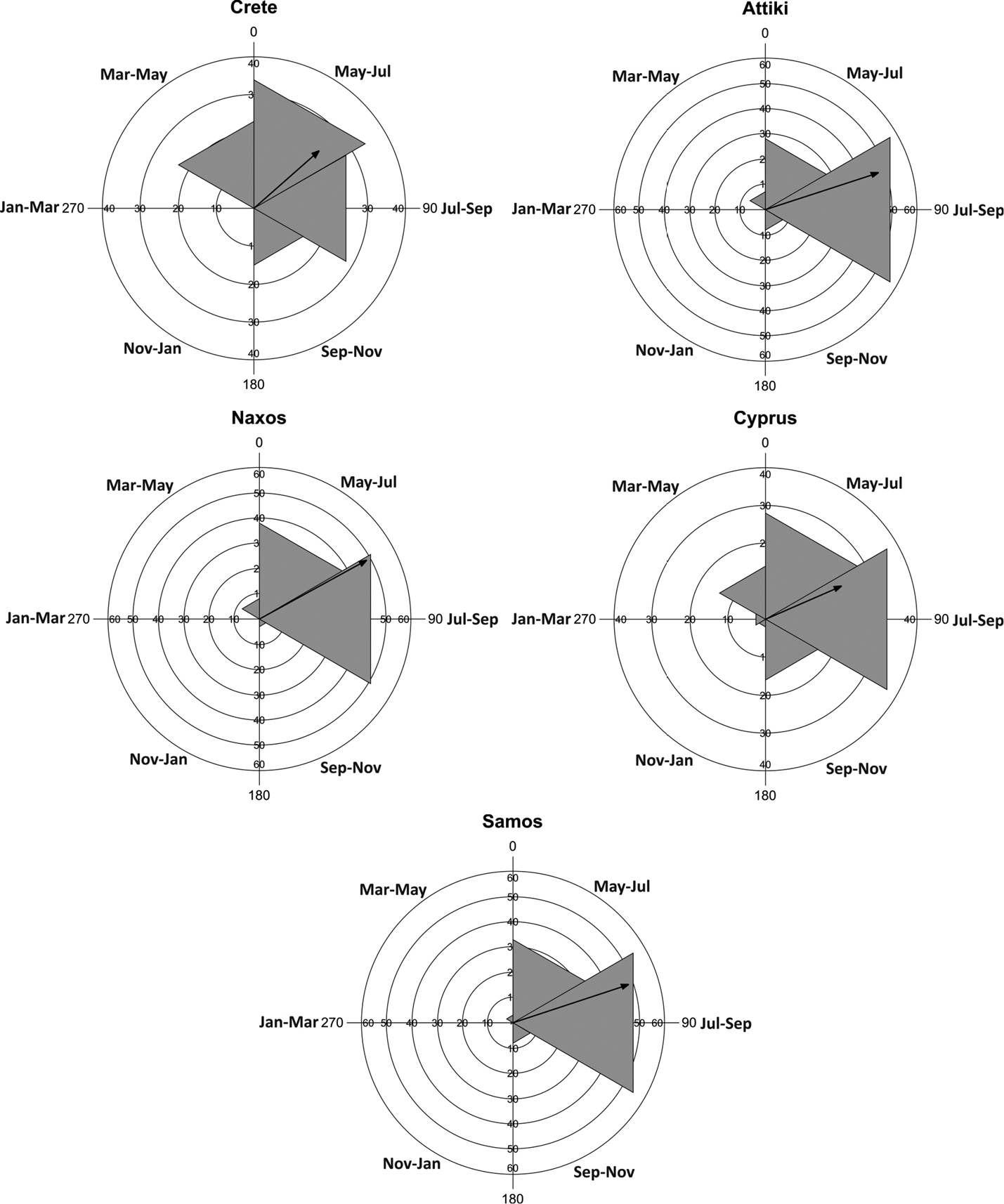
The Watson-William F-test showed that Attiki (F = 1.479, p = 0.225) and Samos (F = 1.647, p = 0.201) were the only sites, where the phenological patterns of scolopendrids were identical in the two years of study, peaking during late summer (July-September). In the other three sites, scolopendrid activity peaked during early summer (May-July) in the first year of study (Crete, Naxos), or over a longer period (May-September) in the second year (Cyprus) (Figure 3). However, the Rayleigh test showed that the cumulative phenological patterns of both species were statistically significant at all sites (Table 3). The mean period of maximal activity of Scolopendra cingulata was midsummer (early-mid July), whereas the activity of Scolopendra cretica peaked during early summer (mid June) (Figure 4). The Watson-William F-test affirmed a statistical difference of the phenology of Scolopendra cretica and the respective pattern of Scolopendra cingulata in Attiki and Samos (Table 4). The same test also showed that the temporal patterns of microhabitat preference did not differ for any of the study sites (1.65E-4 < F < 1.732, 0.19 < p < 0.99).
Activity density (number of individuals/100 trap-days) of Scolopendra cretica (Crete*) and Scolopendra cingulata per sampling period.
Rose diagrams of of circular analysis of abundance of scolopendrids during the whole study. The angles represent the bimonthly sampling intervals. The length of the mean vector (r) is a measure of concentration of data around the year.
Average catchability (number of individuals per trap per 2 years) in each of the three microhabitat types in the five sampling sites. o: open field, sv: scarce vegetation, dv: dense vegetation and litter cover.
| Crete | Attiki | Naxos | Cyprus | Samos | |
|---|---|---|---|---|---|
| o | 2.111 | 9.204 | 4.750 | 4.604 | 13.417 |
| sv | 3.000 | 5.458 | 5.000 | 2.516 | 15.932 |
| dv | 3.008 | 7.150 | 4.313 | 3.100 | 21.000 |
Circular statistics results for temporal activity patterns of scolopendrids in the five study sites.
| Crete | Attiki | Naxos | Cyprus | Samos | |
|---|---|---|---|---|---|
| Mean Vector (µ) | 48.422° | 71.891° | 61.115° | 66.587° | 71.625° |
| Length of Mean Vector (r) | 0.574 | 0.788 | 0.807 | 0.548 | 0.806 |
| Median | May–Jul | Jul–Sep | Jul–Sep | Jul–Sep | Jul–Sep |
| Concentration | 1.407 | 2.721 | 2.954 | 1.312 | 2.931 |
| Circular Variance | 0.426 | 0.212 | 0.193 | 0.452 | 0.194 |
| Standard Error of Mean | 6.376° | 3.913° | 3.711° | 6.758° | 3.729° |
| Rayleigh Test (Z) | 32.943 | 62.058 | 65.172 | 30.004 | 64.887 |
| Rayleigh Test (p) | < 1E-12 | < 1E-12 | < 1E-12 | < 1E-12 | < 1E-12 |
Watson-William F-test results on the comparison of temporal activity patterns during the whole study. Significant differences are shown in bold type.
| Attiki | Naxos | Cyprus | Samos | |||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | |
| Crete | 9.122 | 0.003 | 2.764 | 0.098 | 3.706 | 0.056 | 9.185 | 0.003 |
| Attiki | 3.22 | 0.074 | 0.442 | 0.507 | 0.002 | 0.965 | ||
| Naxos | 0.485 | 0.487 | 3.198 | 0.075 | ||||
| Cyprus | 0.41 | 0.523 | ||||||
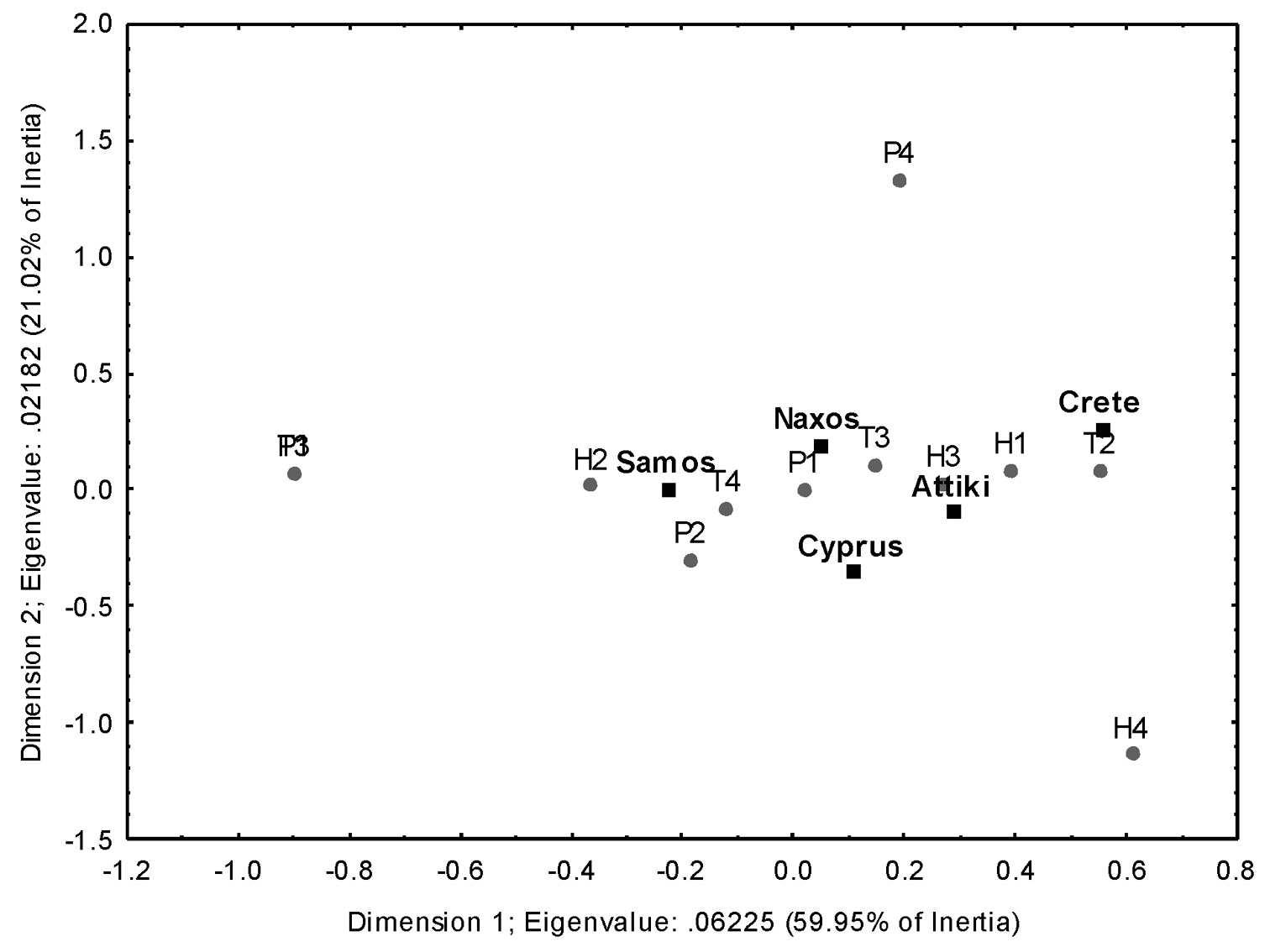
Correspondence analysis (total chi-square = 167.98, d.f. = 44, p < 0.001) showed that there was no differentiation in the influence of abiotic factors on the activity of both species at all five sampling stations (Figure 5). The abundance of both the species was maximal under very low average precipitation (0–2.6 mm), low average air relative humidity (46.4%–59.3%), and high average air temperature (Scolopendra cingulata: 25.2–25.5°C; Scolopendra cretica: 23.6°C).
Correspondence analysis plot. T1–T4: air temperature (in °C) classes (8.43 ≤ T1 < 14.24 ≤ T2 < 20.05 ≤ T3 < 25.86 ≤T4 < 31.67); H1–H4: air relative humidity (%) classes (44.67 ≤ H1 < 52.78 ≤ H2 < 60.89 ≤ H3 < 69 ≤ H4 < 77.12); P1–P4: precipitation (in mm) classes (0 ≤ P1 < 39.33 ≤ P2 < 78.66 ≤ P3 < 117.99 ≤ P4 < 157.32).
Scolopendra cretica and Scolopendra cingulata are among the dominant arthropod species in the Aegean and continental Greece (Scolopendra cretica only in Crete and its adjacent islets) (
The correspondence of the number of active scolopendrids with high air temperature, low air relative humidity and almost zero precipitation implies that Scolopendra cretica and Scolopendra cingulata are thermophilous and xerophilous species. This is in agreement with
We thank Artemis Katsadoura, Myrto Pyrounaki and Eleni Rigopoulou for their kind help to sort out and measure specimens collected in the field. We are grateful to John Lewis, Antony Barber and Ivan Tuf for valuable comments on a previous draft.
Climate data per period in the five sampling sites. T: average air temperature (in °C), H: average air relative humidity (%), P: average precipitation (in mm).
| Crete | Attiki | Naxos | Cyprus | Samos | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | H | P | T | H | P | T | H | P | T | H | P | T | H | P | |
| May 06 - Jul. 06 | 23.6 | 51.2 | 0.3 | 23.3 | 55.9 | 10.6 | 23.8 | 52.3 | 2.5 | 23.1 | 68.8 | 0.5 | 24.1 | 59.6 | 0.5 |
| Jul. 06 - Sept. 06 | 28.6 | 45.4 | 0.0 | 28.6 | 46.3 | 0.3 | 29.1 | 44.7 | 2.1 | 27.4 | 72.8 | 0.0 | 29.3 | 46.6 | 4.0 |
| Sept. 06 - Nov. 06 | 22.4 | 71.3 | 21.8 | 20.5 | 76.8 | 54.4 | 22.0 | 69.8 | 33.2 | 23.8 | 77.1 | 35.5 | 23.2 | 62.1 | 54.0 |
| Nov. 06 - Jan. 07 | 16.5 | 77.0 | 35.8 | 11.1 | 93.9 | 70.7 | 13.3 | 79.0 | 64.5 | 14.2 | 83.8 | 25.3 | 13.8 | 70.9 | 102.0 |
| Jan. 07 - Mar. 07 | 12.4 | 88.4 | 45.1 | 9.2 | 92.0 | 68.1 | 12.1 | 76.6 | 75.3 | 12.2 | 85.6 | 68.3 | 12.4 | 71.6 | 89.9 |
| Mar. 07 - May 07 | 15.2 | 64.3 | 21.1 | 14.4 | 72.3 | 24.2 | 17.2 | 64.2 | 39.4 | 17.6 | 68.5 | 40.3 | 19.0 | 58.5 | 11.3 |
| May 07 - Jul. 07 | 23.6 | 51.6 | 1.5 | 24.3 | 53.4 | 20.2 | 25.9 | 59.0 | 12.7 | 25.9 | 58.2 | 22.1 | 28.1 | 57.6 | 22.3 |
| Jul. 07 - Sept. 07 | 28.9 | 45.1 | 1.0 | 29.8 | 46.4 | 0.0 | 29.4 | 58.7 | 3.0 | 31.6 | 45.8 | 0.0 | 31.7 | 53.1 | 0.0 |
| Sept. 07 - Nov. 07 | 22.0 | 72.3 | 17.2 | 20.9 | 72.3 | 31.3 | 23.3 | 64.4 | 46.6 | 27.1 | 64.9 | 0.1 | 26.2 | 65.9 | 35.5 |
| Nov. 07 - Jan. 08 | 16.0 | 79.7 | 35.3 | 11.5 | 78.4 | 69.3 | 13.5 | 67.6 | 96.1 | 19.4 | 70.1 | 75.5 | 16.6 | 80.1 | 155.4 |
| Jan. 08 - Mar. 08 | 11.2 | 72.1 | 46.1 | 8.4 | 77.7 | 61.0 | 10.4 | 68.5 | 157.3 | 16.2 | 61.2 | 33.3 | 10.6 | 81.4 | 72.3 |
| Mar. 08 - May 08 | 17.9 | 71.9 | 16.4 | 15.4 | 66.2 | 52.5 | 16.5 | 61.3 | 102.2 | 22.8 | 63.6 | 7.8 | 17.0 | 75.6 | 50.5 |